
T
 T |
 |
Rapid Tests for HIV Antibody
Bernard M Branson, MD |
Share this Paper with a Colleague
Abstract
Rapid HIV tests are widely used in resource-poor settings, especially in developing countries. The need for immediate HIV test results to make treatment decisions and to assist with prevention strategies portends their increased use in developed countries as well. Available data on the characteristics and performance of individual test devices are summarized from peer reviewed journals and conference abstracts. Data from test manufacturers were not included unless corroborated by independent evaluations. Rapid HIV tests demonstrate sensitivities and specificities comparable to those of enzyme-linked immunoassays (ELISAs) currently used for screening. Algorithms comprised of a combination of two or more rapid tests produce HIV test results with predictive values comparable to those of the ELISA-Western blot combination. Rapid HIV tests offer additional advantages of low cost and same-day results and are likely to gain increasing acceptance for HIV screening and diagnosis in both developed and developing countries.
Keywords
HIV antibody testing, rapid serological assays, alternative confirmatory strategies
Text
Voluntary human immunodeficiency virus (HIV) antibody testing and counseling services were initiated in March 1985, shortly after the introduction of the enzyme-linked immunoassay (ELISA) for the screening of donated blood. Initially, counseling and testing were intended to provide an alternative to the donation of blood as a means for high-risk persons to determine their HIV status. At that time, little was known about the prevalence and natural history of HIV infection. The benefit of screening blood to prevent HIV transmission from transfusions was clear, but the potential for false-positive results from the use of screening tests in low-prevalence populations raised questions about the usefulness of HIV antibody tests for screening [1]. The paradigm for HIV testing thus evolved to meet the requirements imposed by the need to protect the blood supply: tests with high sensitivity, suitable for batch processing of high volumes of specimens in centralized laboratories with specialized equipment.
The potential personal, medical, and public health benefits of testing for HIV antibody soon became clear [2]. The U.S. Public Health Service issued guidelines recommending ready access to HIV testing for persons who practiced high-risk behaviors [3]. Continued concerns about false-positive screening results [4] led to the implementation of a sequential two-test algorithm, comprising an ELISA screening test followed by Western blot or immunofluorescence assay as a supplemental test, to confirm HIV positively. The U.S. Public Health Service recommended that no positive test results be given to patients until the screening test had been repeatedly reactive on the same specimen and the supplemental test had been used to validate those results [5]. The recommended tests require specialized equipment and technical expertise, and they cannot be completed in less than 24 hours. In practice, given the time necessary to transport specimens to a laboratory, perform the tests in batches, and transmit test results, tested persons typically must wait 1-2 weeks before they make a second visit to learn their test results.
ELISA and Western blot were not feasible for small laboratories in many developing countries where resources are limited and electricity may not be consistently available. These tests require many hours to perform, refrigeration, and sophisticated, expensive equipment [6]. A number of simple, rapid assays emerged to meet the demand in such countries both for blood screening and voluntary testing [7-11]. Numerous studies demonstrated that alternative confirmatory strategies using algorithms with combinations of screening tests produced reliable results, comparable to those of the standard ELISA and Western blot [12-15], and the United Nations Programme on HIV/AIDS - World Health Organization (WHO) currently recommends the routine use of combinations of screening tests for HIV screening, surveillance, and diagnosis (Table 1) [16,17].
Table 1
| Objective | Prevalence | Strategy | |
| Blood Screening | All | 1 | |
| Surveillance | >10% | 1 | |
| <10% | 2 | ||
| Diagnosis | Signs/symptoms | >30% | 1 |
| <30% | 2 | ||
| Diagnosis: | Asymptomatic | >10% | 2 |
| <10% | 3 | ||
| Strategy 1: | Single screening assay. Reactive test is considered positive. |
| Strategy 2: | Two screening assays. If initial test is reactive, test is repeated with second assay. Specimen considered positive only when both assays are reactive. |
| Strategy 3: | Three screening assays. Specimen considered positive only when all three assays are reactive. |
Screening with combinations of rapid HIV tests proved to be less expensive than the ELISA/Western blot algorithm [15], and also made it possible to offer same-day test results. The lower cost made voluntary counseling and testing more feasible for developing countries, and availability of same-day results greatly increased the number of persons who learned their serostatus after testing [18,19]. Providers and clients reported high levels of satisfaction with rapid HIV tests [20].
Although more than 60 rapid HIV tests have been developed and used in various countries, only 2 have received approval from the Food and Drug Administration (FDA) for use in the United States. The first, Recombigen HIV-1 LA [21], was a latex agglutination test. As is true for many other agglutination tests, even technicians with extensive training had difficulty distinguishing reactive test results from the background granularity of the latex particles [11], and Recombigen was withdrawn from the U.S. market because of poor performance. Only one rapid test, SUDS (Single Use Diagnostic System for HIV-1), remains commercially available in the United States, and few are in use in other developed countries [22].
Four findings mandate the increased use of rapid HIV antibody diagnostics both in developing and developed countries for the benefit of public health [23]. First, antiretroviral therapy reduces occupational HIV transmission after percutaneous exposures [24] and reduces vertical transmission when used intra- or postpartum [25]. Access to immediate HIV test results could improve the judicious application of prophylactic regimens [26,271. Second, many persons who are tested for HIV, including those who are HIV-infected, never receive their test results. [28-31]. Several studies suggest that persons who are aware they are HIV-infected adopt behaviors that make their transmission of HIV infection less likely [32-35], and rapid tests can substantially increase the number of persons who receive their test results [20,36,37]. Third, HIV infection in many persons who seek health care services remains undiagnosed P8-40]; rapid HIV tests could substantially assist with identifying these persons and providing them with essential medical and prevention services [40-44]. Finally, persons who are aware of their serostatus and ask about that of potential sex partners are very unlikely to choose a sex partner of opposite status [45]. The use of rapid tests as part of prevention strategies that promote the need for awareness of one's own and one's partner's infection status could reduce the sexual transmission of HIV considerably [46-50].
Assay formats
Most rapid assays are in kit form that requires no other reagent, and many require no other specialized equipment. The three most common generic assay formats (Fig. 1) use particle agglutination, membrane immunoconcentration (flow-through) devices, or immunochromatographic (lateral-flow) strips. Particle agglutination assays typically require 10 to 60 minutes or more and must be used with serum or plasma. When a patient specimen containing HIV antibodies is mixed with minute HIV antigen-coated latex particles, cross linking occurs and results in agglutination. Some devices enhance the visual agglutination reaction by using small, channeled, clear plastic cassettes. Flow of the specimen-particle mixture through narrowed areas in the channels promotes agglutination. Detection of weak agglutination can be difficult, and readers have been developed for some tests to reduce the inaccuracy introduced by subjective interpretation. The reagents often require refrigeration, and costs range from US$2 to $4 per test.
| Figure 1 - Part 1 |
Figure 1 - Part 2 |
|
Agglutination Device |
Flow-Through Device |
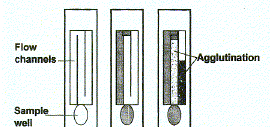 |
 |
| Figure 1 - Part 3 |
|
Lateral-Flow Device |
 |
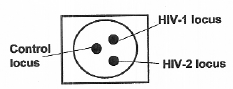
Membrane immunoconcentration devices employ solid-phase capture technology, which involves the immobilization of HIV antigens on a porous membrane. The specimen flows through the membrane and is absorbed into an absorbent pad. A dot or a line visibly forms on the membrane when developed with a signal reagent (usually a colloidal gold or selenium conjugate). Some tests allow the differentiation of HIV-1 from HIV-2 by applying antigens from these viruses to different sites on the membrane: The flow-through tests require several steps for the addition of specimen, wash buffers, and signal reagent, and they can usually be performed in 5 to 15 minutes. Most are used with serum or plasma, though some are equipped with a filter to allow the use of whole-blood specimens. The devices or reagents typically require refrigeration. Costs range from US$4 to $8 per test.
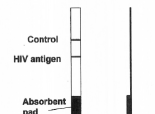
Immunochromatographic strips, the most recent development, potentially require only one step and incorporate both antigen and signal reagent into a nitrocellulose strip. The specimen is applied to an absorbent pad from which it is wicked, combines with signal reagent, and migrates through the strip. A positive reaction results in a visual line on the membrane where HIV antigen has been applied. A few of the strip tests also deploy different antigens at different locations to allow differentiation of HIV-1 group M, HIV-1 group O, and HIV-2 antibodies. A procedural control line that detects immunoglobulin G is usually applied to the strip beyond the HIV-antigen line. A visual line at the test and control sites indicates a positive test result, a line only at the control location indicates a negative test result, and the absence of a line at the control site means the test is invalid. Most lateral-flow tests require no additional equipment or refrigeration, and test results can be obtained in less than 15 minutes. Many can be used with whole blood, serum, or plasma, and some can be used with finger-stick specimens, saliva or oral fluids. In some lateral-flow devices, the test strip is encased in a plastic cartridge. Cost of these tests is usually less than US$2.
Two other formats are used less commonly. Autologous red-cell agglutination tests require 5 minutes or less and detect HIV antibodies with a hybrid antigen-antibody reagent, which, when added to the red cells of the patient, agglutinates the patient's own red cells. Immunodot comb assays use a solid plastic matrix with "teeth" attached to one another, to which HIV antigen is fixed to capture HIV antibodies. Patient specimens are placed in wells spaced to accommodate each tooth of the comb device, which allows batch processing. The tests, which require less than 30 minutes to perform, are then developed with a signal reagent. Results for each specimen are visualized as a spot or a dot on the corresponding tooth.
Methods of antigen production (viral lysate, synthetic peptide, recombinant peptide) and the specific combinations of antigens differ with each individual assay. The devices are sometimes made by one company but distributed and sold under several brand names, which leads to confusion and makes it impossible to compile a comprehensive list. Because regulatory requirements and approvals are often minimal compared with those established by the U.S. FDA, it can sometimes be difficult to gauge the sensitivity and specificity of the tests with confidence. Some entrepreneurs use outlets such as the Internet to sell minimally evaluated tests of uncertain quality directly to the public. WHO, through its Programme on Health Technologies, periodically evaluates ELISAs and rapid tests that are available for bulk purchase by the public sector. The tests are performed on a panel of approximately 600 sera of diverse geographic origins and on 8 seroconversion panels [51 ]. Results of these evaluations are available at http://www.who.int/pht. Table 2 describes tests for which performance data are available from independent evaluations and tests for which preliminary data from active investigations show promise.
TABLE 2
|
Manufacturer |
Product |
Principle |
Sensitivity |
Specificity |
Comments |
|
Determine HIV- 1/2/O |
Lateral flow |
97.9-100 |
100 |
Complexity: 1 |
|
|
Abbott Park, Illinois USA |
Store at room temperature |
||||
|
Whole blood, serum |
|||||
|
Retrocell HIV-1/2 |
Red cell |
100 |
100 |
Complexity: 2 |
|
|
agglutination |
Store at 2-8 ° C |
||||
|
SUDS HIV-1 |
Flow through |
97.9-99.9 |
77.4-99.6 |
Complexity: 3 |
|
|
Store at 2-8 ° C |
|||||
|
Agen Biomed |
SimpliRED HIV-1/2 |
Red cell |
99.2 |
87.3 |
Complexity: 2 |
|
Brisbane, Australia |
agglutination |
Store at 2-8 °C |
|||
|
MicroRED HIV-1/2 |
Particle |
98.5 |
99.5 |
Complexity: 2 |
|
|
agglutination |
Store at 2-8 ° C |
||||
|
Bionor A/S |
Bionor HIV-1/2 |
Magnetic |
100 |
98.8 |
Complexity: 3. |
|
Skien, Norway |
beads |
Store at 2-8 ° C |
|||
|
BioRad Laboratories |
Genie II HIV-1/2 |
Flow through |
97.8-100 |
99.7-100 |
Complexity: 2 |
|
Redmond, Washington USA |
Store at 2-8°C |
||||
|
Multispot HIV-1/2 |
Flow through |
99.3-100 |
98.5-100 |
Complexity: 3 |
|
|
Store at 2-8°C |
|||||
|
Cal Test Diagnostics |
Red. Dot HIV-1/2 |
Flow through |
100 |
94.9 |
Complexity: 3 |
|
Los Angeles, California USA |
Store at 2-8°C |
||||
|
Epitope, Inc. |
OraQuick |
Lateral flow |
100 |
100 |
Complexity: 1 |
|
Beaverton, Oregon USA |
Store at room temperature |
||||
|
Whole blood, serum, saliva |
|||||
|
Fujerebio |
Serodia HIV-1/2 |
Particle |
100 |
98 |
Complexity: 3 |
|
Tokyo, Japan |
agglutination |
Store at 2-8 °C |
|||
|
Genelabs Technologies, Inc. |
HIV SPOT-1/2 |
Flow through |
97-99 |
96-99 |
Complexity: 2 |
|
Redwood City, California USA |
Store at room temperature |
||||
|
Sayvon Diagnostics Ltd. |
HIV SAV-1/2 |
Flow through |
97.7 |
96.7 |
Complexity: 2 |
|
Ashdod, Israel |
Store at room temperature |
||||
|
Hepatika Laboratories |
Entebe HIV Dipstick |
Immunodot |
100 |
96.4 |
Complexity: 3 |
|
Mataram, Indonesia |
comb |
Store at 2-8°C |
|||
|
Immunochemical Laboratories |
Dipstick HIV-1/2 |
Immunodot |
100 |
98.2 |
Complexity: 2 |
|
Bangkok, Thailand |
comb |
Store at 2-8°C |
|||
|
J. Mitra & Co. |
HIV Tri-Dot |
Flow through |
99.6 |
99.7 |
Complexity: 3 |
|
New Delhi, India |
Store at 2-8°C |
||||
|
MedMira Laboratories |
MedMira HIV-1/2 |
Flow through |
99.0-100 |
100 |
Complexity: 2 |
|
Halifax, Nova Scotia, Canada |
Store at room temperature |
||||
|
Whole blood, serum |
|||||
|
Orogencis Ltd. |
DoubleCheck HIV-1/2 |
Immunodot |
100 |
99.7 |
Complexity: 2 |
|
Yavne, Israel |
comb |
Store at room temperature |
|||
|
Ortho Diagnostics |
HIVCHEK System 3 |
Flow through |
98.2-100 |
98.8-100 |
Complexity: 3 |
|
New Brunswick, New Jersey USA |
Store at room temperature |
||||
|
Saliva Diagnostic Systems |
Hema-Strip HIV-1/2 |
Lateral flow |
98.8-99:6 |
99.9-100 |
Complexity: 1 |
|
New York, New York USA |
Store at room temperature |
||||
|
Designed for finger stick |
|||||
|
Sero-Strip HIV-1/2 |
Lateral flow |
98.4-99.9 |
99.6-100 |
Complexity: 2 |
|
|
Store at room temperature |
|||||
|
Span Diagnostics |
CombAIDS Visual |
Immunodot |
100 |
88 |
Complexity: 2 |
|
Surat, India |
comb |
Store at 2-8°C |
|||
|
Trinity Biotech |
Capillus HIV-1/2 |
Particle |
98.6-99.9 |
98.2-99.6 |
Complexity: 2 |
|
Bray, Wicklow Ireland |
agglutination |
Store at 2-8°C |
|||
|
SalivaCard HIV |
Flow through |
98.9 |
98.8 |
Complexity: 2 |
|
|
Store at 2-8 °C |
|||||
|
Saliva |
|||||
|
SeroCard HIV |
Flow through |
99.8-100 |
97.9-99.5 |
Complexity: 2 |
|
|
Store at 2-8°C |
|||||
|
UniGold HIV-1/2 |
Lateral flow |
98.6-99.8 |
99.6-100 |
Complexity: 1 |
|
|
Store at 2-8 ° C |
|||||
|
Whole blood, serum |
|||||
|
Universal Healthwatch |
Quix HIV- 1/2/O |
Flow through |
100 |
99.8 |
Complexity: 2 |
|
Columbia, Maryland USA |
Store at 2-8 °C |
||||
|
Whole blood, serum |
|||||
|
Wiener Labratorios |
DIA HIV-1+2 |
Inimunodot |
99.6 |
99.4 |
Complexity: 2 |
|
Rosario, Argentina |
comb |
Store at 2-8 ° C |
Notes to table 2:
Sensitivity and specificity entries with range represent published reports
against multiple HIV-I/2 subtypes; entries with single figure represent data
from a single independent evaluation, usually that of the WHO.
Complexity rating:
Subtype detection
Paradoxically, rapid HIV tests are used most widely in parts of the world where non-B subtypes of HIV-1 group M, group O, and HIV-2 are found, but few systematic evaluations with sufficient numbers of specimens have been conducted to establish the capacity of the assays to detect these strains. Available data suggest that all subtypes of group M are adequately detected but that test performance is more variable with group O and HIV-2 strains [52-54]. Some tests include only HIV-1 antigens and detect only those HIV-2 strains with cross-reacting epitopes; others (e.g., Multispot) reliably detect and differentiate HIV-2 antibodies. Performance with group O strains is similar to that of ELISAs currently in use. Similarly sparse data from seroconversion panels demonstrate the analytic sensitivity of the rapid assays to be comparable to that of ELISAs currently licensed by the FDA in the United States [53,54].
Discussion
The rationale for diagnostic testing has changed from clinical confirmation of suspected HIV disease to the potential for prevention and care afforded by knowing one's HIV status [17]. The HIV testing paradigm developed at the beginning of the epidemic, predicated on exquisite sensitivity, has served well for blood screening but may be less effective for diagnostic and surveillance purposes. A wide range of HIV antibody tests are available. The challenge today is to identify the most suitable assays for a given set of circumstances without compromising the reliability of test results.
Overall test sensitivity or specificity may be improved by using test combinations under one or more decision rules for resolving discordant results. For instance, the sensitivity of a single test can be improved if the combination is considered positive when either constituent test is positive. In this circumstance, the combined sensitivity reflects the best of the sensitivities achieved by either test. The penalty is specificity, which is reduced to the product of the individual specificities [55]. If the algorithm requires that both tests be positive, the combined sensitivity is the sum of the sensitivities of both tests minus 100, less than the sensitivity of either test alone. Despite improved sensitivity and specificity in each new generation of tests, few if any strategies involve only a single test for HIV screening. The usual strategy has been to screen with a low-cost highly sensitive test and then retest positive specimens with a second highly specific test.
Test sensitivity and specificity alone are not sufficient to establish optimal paradigms for HIV screening. Both logistics and economics pose significant challenges to accomplish the three main objectives of HIV antibody testing: (1) screening of donated blood for transfusion safety; (2) diagnosis of infection in individuals; and (3) epidemiologic surveillance of HIV prevalence. As examples, a single HIV screening test may be appropriate in some resource-poor settings if the alternative is no HIV testing at all [56]; initiating testing even when the full diagnostic algorithm cannot be completed can increase the number of persons who ultimately learn their HIV status because persons may be more likely to pursue further testing when advised of suspicious initial results [57].
As is true of any standard, the gold standard for HIV testing must incorporate the application for which it is intended. For gold itself, 24 karat is the standard for metallic purity, but a 14-karat alloy is used in jewelry because of its hardness and ability to retain shape. By a similar analogy, it is increasingly necessary to design alternative algorithms for HIV testing that take into account the many dimensions of the applications to personal and public health. Evidence suggests that many of the newer rapid HIV tests, which continue to improve, already perform as well as the ELISA and Western blot [58]. Although each test fails to detect antibody in occasional samples, combination-test algorithms can be employed which are as sensitive and specific as the ELISA/Western blot combination. It will be necessary to collect large amounts of data from diverse populations in settings of intended use to validate rapid tests against the standards with which we have become comfortable. While these evaluations are being conducted, it should be possible to perform screening with algorithms consisting of two or more rapid tests used simultaneously (with yet another test to resolve discordant results) so that individuals and public health can reap the benefits of newer technologies with little risk of unreliable results. Given the fast pace of development of rapid HIV tests, it is likely that such evaluations will need to be repeated frequently for the foreseeable future.
Acknowledgements
The author gratefully acknowledges Niel T. Constantine, Ph.D., University of Maryland Institute of Human Virology; Mark Rayfield, Ph.D., Centers for Disease Control and Prevention, Division of AIDS, STD, and TB Laboratory Research; and Milton R. Tam, Ph.D., Program for Appropriate Techonology in Health, for information on specific HIV tests included in Table 2, and Marie Morgan for invaluable assistance in preparation of the manuscript.
References
1. Meyer KB, Pauker SG. Screening for HIV: Can we afford the false positive rate? [editorial]. N Engl J Med 1987;317: 238-41.
2. Rhame FS, Maki DG. The case for wider use of testing for HIV infection [editorial]. N Engl J Med 1989;320:1248-54.
3. Public Health Service guidelines for counseling and antibody testing to prevent HIV infection and AIDS. MMWR Morb Mortal Wkly Rep 1987; 36: 509-15.
4. Tu XM, Litvak E, Pagano M. Issues in human immunodeficiency virus (HIV) screening programs. Am J Epidemiol 1993; 136: 244-55.
5. Interpretation and use of the Western blot assay for serodiagnosis of human immunodeficiency virus type 1 infections. MMWR Morb Mortal Wkly Rep 1989; 38 (No. S-7): 1-7.
6. Branson BM. Assessing diagnostic technologies marketed to less industrialized countries. Journal of International Association of Physicians in AIDS Care 2000; February:28-30.
7. Quinn TC, Riggin CH, K1ineRL, et al. Rapid latex agglutination assay using recombinant envelope polypeptidefor the detection of antibody to the HIV. JAMA 1988; 260: 510-3.
8. Constantine NT, Fox E, Abbatte EA, et al. Diagnostic usefulness of five screening assays for HIV in an east African city where prevalence of infection is low. AIDS 1989; 3:313-7.
9. Spielberg F, Kabeya CM, Ryder RW, et al. Field testing and comparative evaluation of rapid, visually readscreening assays for antibody to human immunodeficiency virus. Lancet 1989; i: 580-4.
10. Van kerckhoven I, Vercauteren G, Piot P, et al. Comparative evaluation of 36 commercial assays for detecting antibodies to HIV. Bull World Health Organ 1991; 69: 753-60.
11. Malone JD, Smith ES, Sheffield J, et al. Comparative evaluation of six rapid serological tests for HIV-1antibody. J Acquir Immune Defic Syndr 1993; 6: 115-9.
12. Mitchell SW, Mboup S, Mingle J, et al. Field evaluation of alternative HIV testing strategy with a rapid immunobinding assay and an agglutination assay. Lancet 1991; 337: 1328-30.
13. Urassa WK, Bredberg-Raden U, Mbena E, et al. Alternative confirmatory strategies in HIV-1
antibody testing. J Acquir Immune Defic Syndr 1992; 5: 170-6.14. Brattegaard K, Kouadio J, Adom ML, et al. Rapid and simple screening and supplemental testing for HIV-1 and HIV-2 infections in west Africa. AIDS 1993; 7: 8835.
15. Stetler HC, Granade TC, Nunez CA, et al. Field evaluation of rapid HIV serologic tests for screening and confirming HIV-1 infection in Honduras. AIDS 1997; 11: 369-75.
16. Joint United Nations Programme on HIV/AIDS (UNAIDS).
Revised recommendations for the selection and use of HIV antibody tests. Wkly Epidemiol Rec 1997; 72: 81-88.17. Joint United Nations Progamme on HIV/AIDS. The importance of simple/rapid assays in HIV testing. Wkly Epidemiol Rec 1998; 73: 321-8. PDF
18. McKenna SL, Muyinda GK, Roth D, et al. Rapid HIV testing and counseling for voluntary testing centers in Africa. AIDS 1997; 11(suppl l ): S 103-S 110.
19. Kassler WJ, Alwano-Edyegu MG, Marum E, et al. Rapid HIV testing with same-day results: A field trial in Uganda. Int J STD AIDS 1998; 9:134-8.
20. Downing RG, Otten RA, Manun E. Optimizing the delivery of HIV counseling and testing services: The Uganda experience using rapid HIV antibody test algorithms. J Acquir Immune Defic Syndr 1998; 18: 384-8.
21. Starkey CA, Yen-Lieberman B, Proffitt MR. Evaluation of the Recombigen HIV-1 latex
agglutination test. J Clin Microbiol 1990; 28: 819-22.22. Spielberg F, Kassler WJ. Rapid testing for HIV antibody: A technology whose time has come. Ann Int Med 1996; 125:09-11.
23. Kane B. Rapid testing for HIV: Why so fast? [editorial]. Ann Intern Med 1999; 131: 4813.
24. Case-control study of HIV seroconversion in health-care workers after percutaneous exposure to HIV-infected blood-France, United Kingdom, and United States, January 1988 - August 1994. MMWR Morb Mortal Wkly Rep 1995; 44: 929-33.
25. Wade NA, Birkhead GS, Warren BL, et al. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N Engl J Med 1998; 339: 1409-14.
26. Crobman WA, Garcia PM.
The cost-effectiveness of voluntary intrapartum rapid human immunodeficiency virus testing for women without adequate prenatal care. Am J Obstet Gynecol 1999; 181: 1062-71.27. Minkoff H, O'Sullivan MJ. The case for rapid HIV testing during labor [editorial]. JAMA 1998; 279: 1743-4.
28. Centers for Disease Control and Prevention. Update: HIV counseling and testing using rapid tests-United States, 1995. MMWR Morb Mortal Wkly Rep 1998; 47: 211-5.
29. Valdiserri RO, Moore M, Gerber AR, et al. A study of clients returning for counseling after HIV testing: Implications for improving rates of return. Public Health Rep 1993; 108:12-8.
30. Irwin KL, Valdeserri RO, Holmberg SD. The acceptability of voluntary HIV antibody testing: A decade of lessons learned. AIDS 1996; 10: 1707-17.
31. Tao G, Branson BM, Kassler WJ, et al. Rates of receiving HIV test results: Data from the U.S. National Health Interview Survey for 1994 and 1995. J Acquir Immune Defic Syndr 1999; 22: 395-400.
32. McCusker J, Stoddard AM, Mayer KH, et al. Effects of HIV antibody test knowledge on subsequent sexual behaviors in a cohort of homosexually active men. Am J Public Health 1988; 78: 462-7.
33. Godfried JP, van Griensven MS, Ernest MM, et al. Impact of HIV antibody testing on changes in sexual behavior among homosexual men in the Netherlands. Am J Public Health 1988; 78:1575-7.
34. Cleary PD, Van Devanter N, Rogers TF, et al. Behavior changes after notification of HIV infection. Am J Public Health 1991; 81: 1586-90.
35. Often MW, Zaidi AA, Wroten JE, et al. Changes in sexually transmitted disease rates after HIV testing and posttest counseling, Miami, 1988 to 1989. Am J Public Health 1993; 83: 529-33.
36. Kassler WJ, Dillon BA, Haley C, et al. On-site, rapid HIV testing with same-day results and counseling. AIDS 1997; 11: 1045-51'.
37. Farnham PG, Gorsky RD, Holtgrave DR, et al. Counseling and testing for HIV prevention: Costs, effects, and cost-effectiveness of more rapid screening tests. Public Health Rep 1996; 3: 44-53.
38. Clark SJ, Kelen GD, Henrard DR, et al. Unsuspected primary human immunodeficiency virus type 1 in saeronegative emergency department patients. J Infect Dis 1994; 170: 194-7.
39. Wiley DJ, Frerichs RR, Ford WL, et al. Failure to learn human immunodficiency virus test results in Los Angeles public sexually transmitted disease clinics. Sex Trans Dis 1998; 25: 342-5.
40. Irwin K, Olivo N, Schable CA, et al. Performance characteristics of a rapid HIV antibody assay in a hospital with a high prevalence of HIV infection. Ann Intern Med 1996; 125: 471-5.
41. Kelen GD, Bennecoff TA, Kline R, et al. Evaluation of two rapid screening assays for the detection of human immunodeficiency virus-1 infection in emergency department patients. Am J Emerg Med 1991; 9: 416-20.
42. Kelen GD, Shahan JB, Quinn TC. Emergency department-based HIV screening and counseling: Experience with rapid and standard serologic testing. Ann Emerg Med 1999; 33:147-54.
43. Francis DP, Singleton JA. Reporting of HIV-1 infection through provision of essential services [editorial]. J Acquir
Immune Defic Syndr 1992; 6: 285-6.44. Kilmarx PH, Hamers FF, Peterman TA. Living with HIV: Experiences and perspectives of HIV-infected sexually transmitted disease clinic patients after posttest counseling. Sex Trans Dis 1998; January: 28-36.
45. Wiktor SJ, Biggar RJ, Melgye M, et al. Effect of knowledge of human immunodeficiency virus infection statuson sexual activity among homosexual men. J Acquir Immune Defic Syndr 1990; 3: 62-8.
46. Goedert JJ. What is safe sex? Suggested standards linked to testing for human immunodeficiency virus [editorial]. N Engl J Med 1987; 316: 1340-1.
47. Hearst N, Hulley SB. Preventing the heterosexual spread of AIDS: Are we giving our patients the best advice? [editorial]. JAMA 1988; 259: 2428-32.
48. Merson MH, Feldman EA, Bayer R, et al. Rapid self testing for HIV infection. Lancet 1996; 348: 352-3.
49. Dawson JM, Fitzpatrick RM, Reeves G, et al.
Awareness of sexual partners' HIV status as an influence upon high-risk sexual behaviour among gay men. AIDS 1994, 8: 837-41.50. Varghese B, Maher JE, Paterman TA, et al. What's love got to do with it? Quantifying the risk of HIV [abstract]. In: Abstracts of the National HIV Prevention Conference. Atlanta, Georgia: Centers for Disease Control and Prevention; 1999:139.
51. Operational characteristics of commercially available assays to determine antibodies to HIV-1 and/or HIV-2 inhuman sera. Geneva, Switzerland: United Nations Programme on AIDS and World Health Organization. Reports 1-10, 1993-1998.
52. Constantine NT, Zekeng L, Sangare AK, et al. Diagnostic challenges for rapid human immunodeficiency virusassays: Performance using HIV-1 group O, HIV-1 group M, and HIV-2 samples. J Human Virol 1997; 1: 45-51.
53. Dobbs TL, Granade TC, Phillips SK, et al. Ability of current rapid HIV antibody assays to identify sera from individuals infected with HIV-1 Group M subtypes A-E, HIV-1 Group O and HIV-2 [abstract]. In: Program and abstracts of the Thirteenth Annual Conference on Human Retrovirus Testing. San Diego, California: Association of Public Health Laboratories; 1998. PDF
54. Constantine NT. Detection of antibodies to HIV-1 group O by eight screening and four confirmatory assays [abstract]. In: Program and abstracts of the Fourteenth Annual Conference on Human Retrovirus Testing. Albuquerque, New Mexico: Association of Public Health Laboratories; 1999.
55. Albritton W, Vittinghoff E, Padian NS. Human immunodeficiency virus testing for patient-based and population-based diagnosis. J Infect Dis 1996; 174(suppl 2): S 176-81.
56. Wilkinson D, Wilkinson N, Lombard C, et a1 On-site HIV testing in resource-poor settings: Is one rapid test enough? AIDS 1997; 11: 377-81.
57. Tao G, Kassler WJ, Branson BM, et al. Home collection kits for HIV testing: Evaluation of three strategies for dealing with insufficient dried blood specimens. J Acquir Immune Defic Syndr 1997; 15: 312-7.
58. Branson B, Woehrle T, Fridlund C, et al. Performance of newer whole-blood rapid tests for HIV antibody [abstract]. In: Program and abstracts of the Association of Public Health Laboratories Conference on Laboratory Aspects of Human Retrovirus & Hepatitis C Testing. Charlotte, North Carolina: Association of Public Health Laboratories; 2000.
Disclaimer
The use of trade names, commercial products, or organizations is for identification purposes only and does not imply endorsement by the U.S. government.
Correspondence
Bernard M. Branson, M.D.
Division of HIV/AIDS Prevention - Surveillance and Epidemiology
National Center for HIV, STD, and TB Prevention
Centers for Disease Control and Prevention
Atlanta, Georgia USA
Email: Bbranson@cdc.gov
Reprints
National Center for HIV, STD, and TB Prevention
Office of Communications
Mailstop E-06
Centers for Disease Control and Prevention
Atlanta, GA 30333
|
|
|