
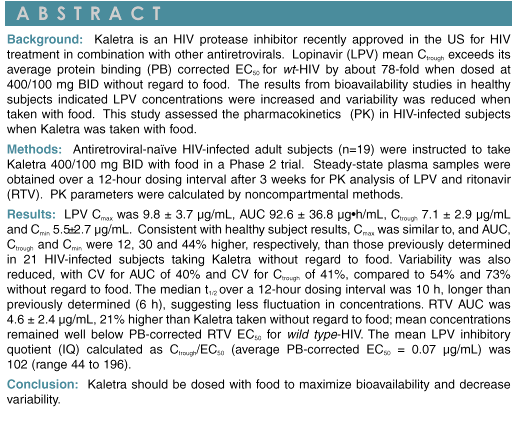
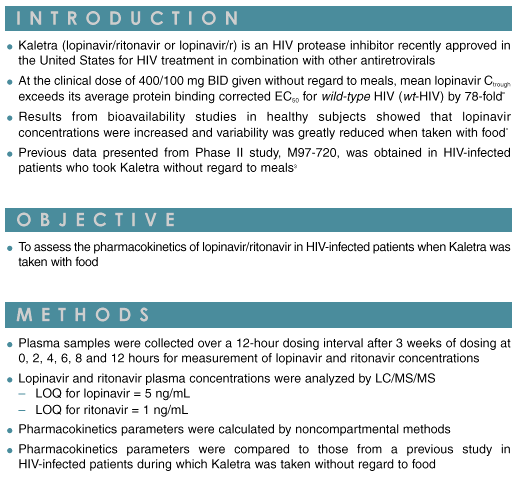
 |
Share this Poster with a Colleague










References:
1. Murphy RL, Brun S, Hicks C, et al. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naïve adults with HIV-1 infection: 48-week results AIDS. 2001;15:1-9.
2. Gustavson L, Lam W, Bertz R, Hsu A, Rynkiewicz K, Ji Q, Ghosh S, Facey I, Bernstein B, Sun E. Assessment of the bioequivalence and food effects for liquid and soft elastic capsule co-formulations of ABT-378/ritonavir (ABT-378/r) in healthy subjects [Abstract 1659]. 40th ICAAC, American Society for Microbiology. Toronto, Canada, September, 2000.
3. Bertz R, Lam W, Brun S, Kumar G. Fields C, Orth K, Jennings J, Hsu A, Granneman GR, Japour A, Sun E. Multiple-dose pharmacokinetics of ABT-378/ritonavir in HIV+ subjects [Abstract 327]. 39th ICAAC, American Society for Microbiology. San Francisco, California, September, 1999.
© 2001 Medical Advocates
for Social Justice
225 West Washington Street, Suite 2200, Chicago, IL 60606
Telephone (312) 419-7289 Facsimile: (312) 419-7151
Email: info@medadvocates.org