
 |
Share this Poster with a Colleague
ABSTRACT
Background: Kaletra is a protease inhibitor recently approved in the US for HIV treatment in combination with other antiretrovirals. Lopinavir (LPV) mean Ctrough exceeds its average protein binding corrected EC50 for wt-HIV by 75-fold when dosed at 400/100 mg BID. The effects of gender, race, weight and age on the pharmacokinetics (PK) of LPV were explored.
Methods: PK data from 7 single-dose co-formulated capsule bioavailability studies in healthy adults were analyzed using ANCOVA. Study, gender and race were factors, age and body weight were covariates in the statistical model. A dose of 400/100 or 400/200 mg LPV/ritonavir was taken with food; the study factor in the model accounted for differences in dose. PK parameters were log-transformed Cmax, AUC and C24/Cmax (effective t1/2). Interaction terms were not significant; none were included in final model. Race effects were compared pairwise.
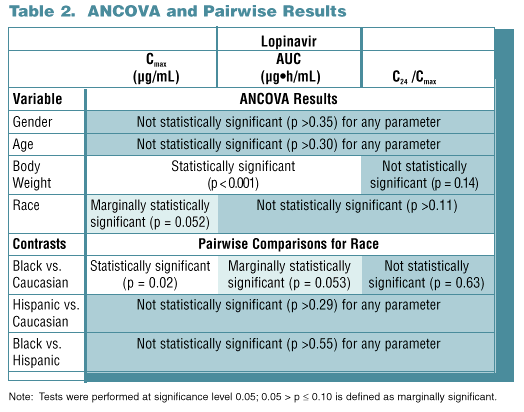
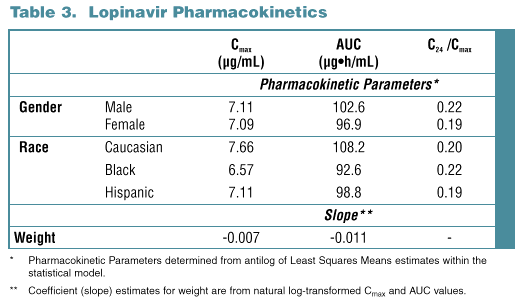
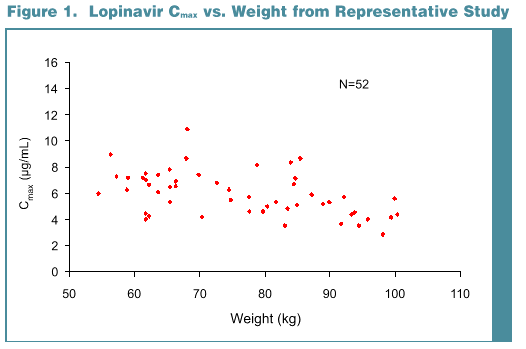
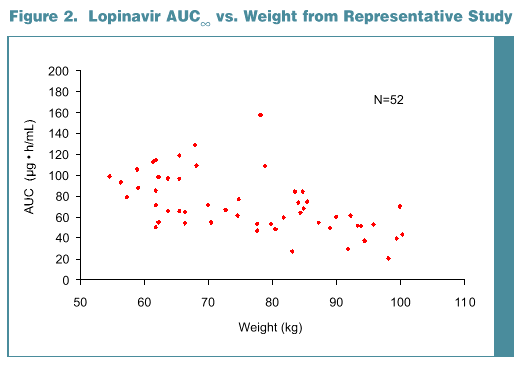
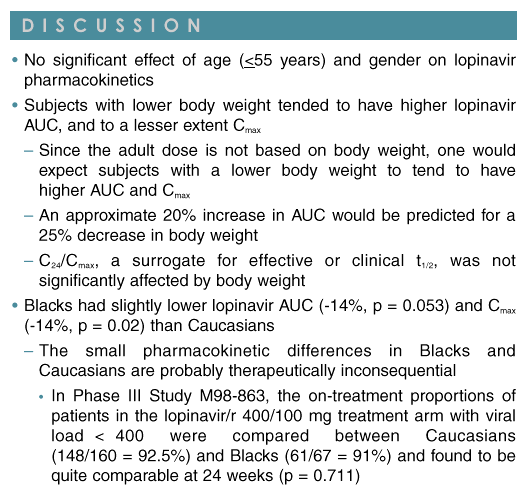
Results: Data from 194 subjects were analyzed, 50 female and 144 male. Most were Caucasian (157), 17 Hispanic and 20 Black. Subject age ranged from 18 to 55 yr (mean 31.6) with a weight range of 54.5 to 100.3 kg (mean 75.7). No statistically significant effect of gender (p >0.35) or age (p >0.30) was noted for any PK parameter. Weight was statistically significant for both AUC and Cmax (p <0.001), but not effective t1/2 (p=0.14). Subjects with lower body weight tend-ed to have higher LPV Cmax and AUC; an approximate 20% increase in AUC would be predicted for a 25% decrease in body weight. Race was marginally significant for Cmax (p=0.052). Blacks had slightly lower LPV AUC (-14%, p=0.053) and Cmax (-14%, p=0.02) than Caucasians. Differences are probably not clinically relevant as reflected by similar efficacy results for Blacks and Caucasians in a large Phase 3 trial, 863.
Conclusion: Of the demographic factors explored in a healthy adult population, body weight was the most significant predictor of LPV PK after single doses of Kaletra.
INTRODUCTION
OBJECTIVE
METHODS
– Single-dose capsule co-formulations of lopinavir/r
- Lopinavir/r 400/100 mg (4 studies, N=144)
- Lopinavir/r 400/200 mg (3 studies, N=50)
– Non-fasting conditions



© 2001 Medical Advocates
for Social Justice
225 West Washington Street, Suite 2200, Chicago, IL 60606
Telephone (312) 419-7289 Facsimile: (312) 419-7151
Email: info@medadvocates.org