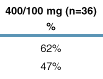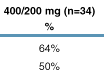|
SHARE THIS POSTER WITH A COLLEAGUE
BACKGROUND
ABT-378 (lopinavir) is a novel HIV
protease inhibitor (PI) that is co-formulated with ritonavir, an inhibitor of
cytochrome P450 3A. It is exquisitely sensitive to pharmacokinetic enhancement
by ritonavir, resulting in substantially increased ABT-378 drug exposure, even
at low ritonavir doses. At the dosage selected for phase III clinical trials,
400 mg ABT-378/100 mg ritonavir BID (as 3 co-formulated capsules BID), ritonavir
concentrations are below those required for antiviral activity. 1
The mean ABT-378 C trough /EC 50 ratio (Inhibitory Quotient or IQ)
for wild-type HIV is >75 when dosed at 400/100 mg BID, potentially
providing a pharmacologic barrier to the emergence of viral resistance and
activity against resistant virus.1 In contrast, the IQs
for currently available Pls whether dosed as single PIs or in conjunction with
ritonavir, range from 1-26 (Figure 1), based on the EC 50 values determined in
the presence of 50% human serum. 2 C trough
values in HIV+ patients for currently available PIs are derived from published
sources including package inserts. 3-14
The efficacy and safety of Kaletra (lopinavir/ritonavir,
formerly known as ABT-378/r) are currently being studied in HIV-infected
patients, both antiretroviral-naïve and PI-experienced. The M97-765 study is an
ongoing phase II trial of ABT-378/r in combination with nevirapine and 2 NRTIs
in single PI-experienced, NNRTI-naïve patients. Results through Week 96 are
presented here.
Figure 1. C
trough
/EC 50
Ratio (Inhibitory Quotient) for PIs Given
Alone or with RTV*
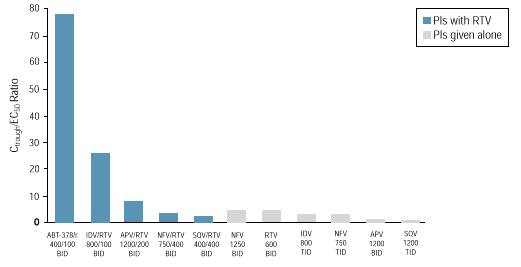
* Contribution of RTV to antiviral
activity not included; regimens shown are those where data in HIV-infected
patients are available; EC 50 values adjusted for protein binding.
METHODS
Entry Criteria
-
Plasma HIV RNA 10 3 –10 5
copies/mL.
-
On treatment with 1 PI and 2
NRTIs for �3 months at study entry.
-
Single PI-experienced (no prior
treatment with any PI, other than the current one, for �6 weeks, and no
prior dual PI therapy).
-
Naïve to at least one NRTI,
with no prior NNRTI experience.
Study Design and Analysis
-
Seventy patients were randomized
to receive ABT-378/r (400/100 mg BID or 400/200 mg BID) in place of their
current PI, in combination with their existing NRTIs.
-
On study day 15, nevirapine (200
mg QD for 14 days then 200 mg BID) was added and NRTIs were changed to
include at least one which was new to the patient (Figure 2).
-
Plasma HIV RNA was quantified
using Roche Amplicor HIV-1 Monitor ™ (lower limit of quantitation [LLQ]
400 copies/mL) and the Roche Amplicor HIV-1 Monitor Ultrasensitive HIV RNA
quantitative PCR, version 1.0 (LLQ 50 copies/mL).
RESULTS
Baseline Characteristics
-
Sixty-three male and 7 female
patients: 66% Caucasian, 24% Black, 7% Hispanic, 3% Asian/Pacific
Islander.
-
Mean age: 40 years (range 22–66).
-
Median plasma HIV RNA: 4.0 log
10 copies/mL (range 2.9–5.8).
-
Median CD4 count: 349 cells/mm 3
(range 72–807).
-
Previous PI-experience included
indinavir (44%), nelfinavir (36%), saquinavir (13%), ritonavir (6%), and
amprenavir (1%).
-
3TC was the most common NRTI
being used at baseline (87%), followed by d4T (56%) and zidovudine (43%),
with a small minority receiving ddI (10%).
Baseline Viral Susceptibility
-
Protease inhibitor phenotypic
susceptibility data were available for 57/70 baseline viral isolates (VIRCO
Antivirogram ® method).
-
Baseline viruses from 63% of
patients (36/57) displayed a > 4-fold loss in susceptibility to
the previous PI, and 32% of patients (18/57) had viruses with >
4-fold loss in susceptibility to three or more PIs (Table 1).
-
Of 56 patients for whom baseline
phenotype for all of the drugs in the previous regimen was available, the
incidence of �4-fold change in EC 50 to one, two or three previous drugs
was 36%, 45%, and 16%, respectively.
-
Nineteen percent of patients
(11/57) had �4-fold reduction in susceptibility to ABT-378 at
baseline.
Table 1. Baseline
Susceptibility to Previous Protease Inhibitor
  
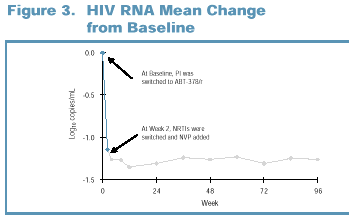
Viral Load Response at Week 2
Viral suppression was
rapid with a marked reduction in HIV RNA during the first two weeks of
therapy. At Week 2, prior to the addition of nevirapine, 80% (56/70) of
patients had a 1 log 10 copies/mL decrease in HIV RNA or achieved a viral load
<400 copies/mL (Figure 3).
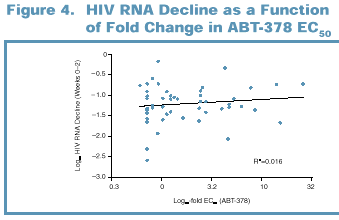
Viral load decline
through Week 2 was independent of the EC 50 of ABT-378 against baseline viral
isolates (range 0.7–26-fold) (Figure 4). This initial decline was also
independent of prior PI experience.
Viral Load Suppression at 96
Weeks
On-treatment analysis
(OT): 81% of patients in the 400/100 mg dose group and 81% of patients in the
400/200 mg dose group had viral load (VL) <400 copies/mL at Week 96 (Figure
5).
Intent-to-treat analysis
(ITT M=F; missing values considered as treatment failure): 61% of patients in
the 400/100 mg dose group and 65% of patients in the 400/200 mg dose group had a
VL <400 copies/mL at Week 96 (Figure 6).
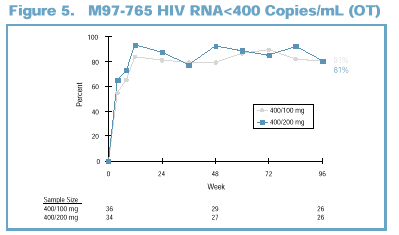 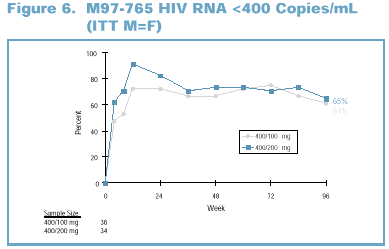
Table 2.
Percentage of Patients with Plasma HIV RNA <50 Copies/mL at 96 Weeks
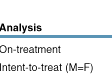 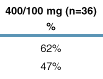 
Viral Load Suppression in Patients
Discontinuing Nevirapine (NVP)
-
3 patients discontinued NVP within 2-4
Weeks after initiation. All 3 patients remained on study through 96 Weeks
and 3/3 had a Week 96 VL <400 copies/mL (3/3 <50 copies/mL).
-
4 additional patients discontinued NVP
between Week 12 and Week 72. One patient discontinued the study at Week 36
due to an AE, and the other 3 patients remained on study through 96 Weeks.
Two of the 3 patients had a Week 96 VL <400 copies/mL (1/2 <50 copies/mL).
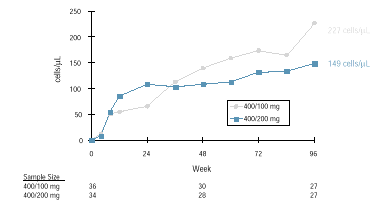
CD4 Cell Response
The mean CD4 cell count
at Week 96 was 575 cells/mm3. (The mean increase from baseline was
188 cells/mm 3 ) (Figure 7).
Differences between dose
groups at Week 96 were not statistically significant.
Figure 7.
M97-765 CD4 Cell Count Mean Change from Baseline
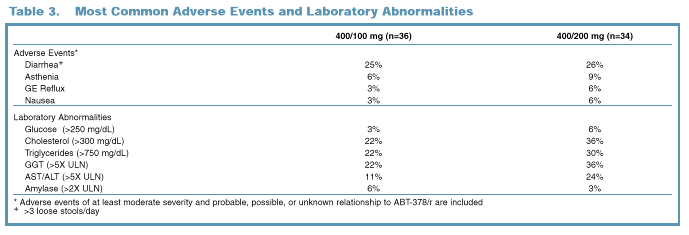
Tolerability
The most common
drug-related adverse events through 96 Weeks were diarrhea and asthenia (Table
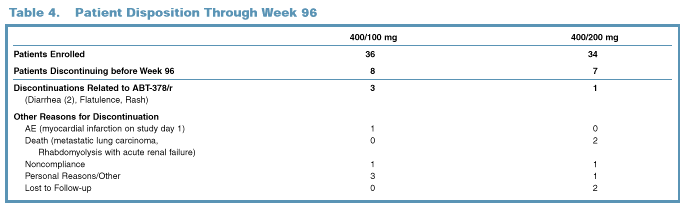
3).
Fifteen
patients discontinued the study through 96 Weeks; of these, only four
discontinuations were due to adverse events related to study drug (Table 4).
No cases of symptomatic
hepatitis attributed to ABT-378/r therapy were reported.
CONCLUSIONS
- ABT-378/r exhibits a potent
and durable antiviral effect through 96 Weeks in PI-experienced patients,
with VL <400 copies/mL in 81% of patients on treatment (ITT M=F: 63%) and
<50 copies/mL in 63% of patients on treatment (ITT M=F: 49%).
ABT-378/r was well
tolerated with only 4/70 patients discontinuing due to adverse events related
to study drug through 96 Weeks.
Viral suppression was
achieved and maintained in the majority of patients, despite high-level
baseline resistance to prior PIs and NRTIs.
Virologic response was
demonstrated during the initial two weeks of the study when the only
alteration to the baseline regimen was substitution of the previous PI with
ABT-378/r. Moreover, of patients who discontinued nevirapine during the course
of this study, 5/7 maintained a viral load <400 copies/mL and 4/7
maintained a viral load <50 copies/mL through 96 Weeks of ABT-378/r
therapy.
While significant viral
suppression was observed in this study of patients with prior PI and NRTI
experience, antiviral activity in antiretroviral-naïve patients receiving
ABT-378/r with d4T and 3TC was higher. 15 This suggests that early use
of ABT-378/r may be associated with optimal outcome.
REFERENCES
-
Bertz R, Lam W, Brun S, et al.
Multiple-dose pharmacokinetics (PK) of ABT-378/ritonavir (ABT-378/r) in HIV+
subjects. 39th Interscience Conference on Antimicrobial Agents and
Chemotherapy, San Francisco, USA, 1999 (abstract 0327).
-
Kempf, DJ, Molla A, Hsu A.
Protease inhibitors as anti-HIV agents. Antiretroviral Therapy, American
Society for Microbiology Press, E DeClerq, editor. In press.
-
Ritonavir Package Insert.
-
Indinavir Package Insert.
-
Amprenavir Package insert.
-
Nelfinavir Package Insert.
-
Saquinavir (Fortavase) Package
Insert.
-
Cohen C, et al. Potent and
convenient Fortovase ™ (SQV) SGC BID regimens in combination with 2
nucleosides or nelfinavir (NFV) plus 1 nucleoside in HIV-1 infected
patients. 12th World AIDS Conference, Geneva, Switzerland, 1998, (abstract
12314).
-
Saag M, et al. Saquinavir
systemic exposure and safety on once daily administration of Fortovase ™
(Saquinavir) soft gel capsules (FTV) in combination with low-dose ritonavir.
39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San
Francisco, USA, 1999, (abstract 330).
-
Shulman N, et al. Ritonavir
intensification in indinavir recipients with detectable HIV RNA levels. 7th
Conference on Retroviruses and Opportunistic Infections, San Francisco, USA
(abstract 534).
-
Piscitelli S, et al. The
addition of a second protease inhibitor eliminates Amprenavir-Efavirenz drug
interactions and increases plasma Amprenavir concentrations. 7th Conference
on Retroviruses and Opportunistic Infections, San Francisco, USA (abstract
78).
-
Mallolas J, et al. A
dose-finding study of once-daily Indinavir/Ritonavir plus Combivir ® in HIV
infected patients. First International Workshop on Clinical Pharmacology of
HIV Therapy, March 2000 (abstract 2.14).
-
Flexner C, et al. Steady-state
pharmacokinetic interactions between ritonavir (RTV), nelfinavir (NFV), and
the nelfinavir active metabolite M8 (AG1402), 12th World Aids Conference,
Geneva (abstract 42265).
-
Cameron DW, et al. Ritonavir and
saquinavir combination therapy for the treatment of HIV infection. AIDS,
1999, 12(2) 213-224.
-
Benson C, King M, Brun S, et al.
ABT-378/ritonavir (ABT-378/r) in antiretroviral-naïve HIV+ patients: 96
weeks. 40th Interscience Conference on Antimicrobial Agents and
Chemotherapy, Toronto, Canada, Sept. 2000 (Poster 546).
ACKNOWLEDGMENTS
- AIDS Research Consortium of
Atlanta Bohn
H, Sullivan M, Enstrom T
- Brigham and Women’s
Hospital Koziol
C
- Cornell Clinical Trials
Unit Sarraco
T, Stroberg T
- Duke University Medical
Center Giner
J, Harmon L
- Infectious Disease
Physicians King
T
- Northwestern
University Bruce
J, Wong A, Donath P
- Pacific Oaks
Research Perry
B, Walker S
- Rush Presbyterian St Luke’s
Medical Center Narkiewicz
E
- San Francisco General
Hospital Raggett
D
- University of
Cincinnati Black
J, Daniel P, Powell T
- University of Colorado Health
Sciences Center Canmann
S, Putnam B, Ray MG
- University of North Carolina
at Chapel Hill Marcus
C, Ngo L
- University of
Pittsburgh Rosener
R
- VIRCO
Hertogs K,
Schel P, Verbiest W
- PPD
Development McCarley
S, Donnelly A, Jackson S, Nicks B
- Abbott
Laboratories Kempf
D, Flanders R, Lindberg M, Yang G, Rode R

Durable
Suppression of HIV+ RNA After Two Years of Kaletra (ABT-378/ritonavir) Therapy
in
Single Protease Inhibitor Experienced Patients
© 2000 Medical Advocates
for Social Justice |