|
Medical Advocates for Social Justice
Conference Poster
|
 |
1st International AIDS Society Conference on HIV Pathogenesis and Treatment.
Buenos Aires,
Argentina -
July 8 - July 11, 2001
Kaletra (ABT-378/ritonavir) in HIV-Infected Children at 72 Weeks.
P. Cahn1, C. Renz5, X. Saez-Lorens2, A. Violari3, P. Gomez4, and E. Sun5for the M98-940 Project Team.
1Fundacion Huesped, Buenos Aires, Argentina, 2Hosp. del Nino, Panama City, Panama, 3Baragwanath Hosp., Johannesburg, South Africa, 4Princess Margaret, Nassau, Bahamas, and 5Abbott Laboratories
|
|
|
|
BACKGROUND
Kaletra (formerly known as ABT-378/r, lopinavir/ritonavir) is a novel HIV protease inhibitor (PI) that has demonstrated significant antiviral activity and
tolerability in clinical trials to date.
Lopinavir is co-formulated with ritonavir, an inhibitor of cytochrome P450 3A. It is uniquely sensitive to pharmacokinetic enhancement by ritonavir, resulting in
substantially increased lopinavir plasma drug exposure, even at low ritonavir doses. At the capsule dosage selected for phase III clinical trials in adults,
400 mg lopinavir/100 mg ritonavir BID, ritonavir concentrations are below those required for antiviral activity.1
The efficacy and safety of Kaletra are currently being studied in HIV-infected adult subjects, both antiretroviral-naïve and PI-experienced. Study M98-940
is a Phase I/II, open-label study of co-formulated Kaletra (liquid) at two doses in combination with reverse transcriptase inhibitors in treatment-naïve
and -experienced pediatric subjects.
METHODS
Objectives
Evaluation of the safety, tolerability, antiviral activity, and pharmacokinetics of co-formulated Kaletra (liquid) in HIV-infected children.
Entry Criteria
- Age: between 3 months and 12 years
- Plasma HIV RNA >400 copies/mL
- No prior NNRTI therapy
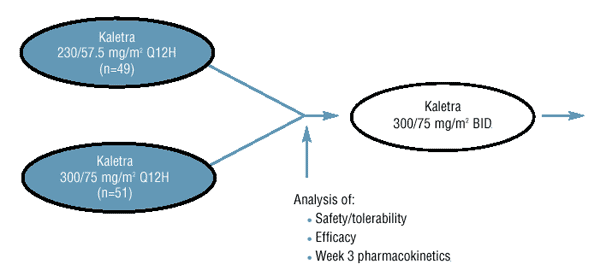
Study Design and Analysis
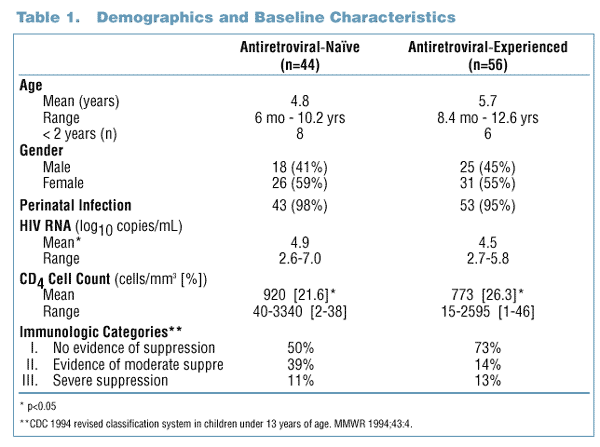
- One hundred (100) antiretroviral-naïve and -experienced pediatric subjects were randomized to receive one of two Kaletra (lopinavir/ritonavir) dosage
levels (230/57.5 mg/m2
Q12H or 300/75 mg/m2
Q12H) selected to approximate the adult drug exposure at 400/100 mg BID.
- Subjects were defined as antiretroviral-naïve if they had received <3 months of prior antiretroviral therapy and <1 week of treatment with 3TC and
antiretroviral-experienced if they had received >3 months of prior antiretroviral therapy or >1 week of treatment with 3TC.
- In addition to Kaletra, antiretroviral-naïve subjects received treatment with d4T and 3TC while antiretroviral-experienced
subjects received treatment
with nevirapine and 1 or 2 NRTIs of the investigator’s choice.
- All subjects were switched to a Kaletra dose of 300/75 mg/m2
following an analysis of safety/tolerability, efficacy and Week 3 pharmacokinetics.
RESULTS
Treatment Experience and Pharmacokinetics
- Of the 56 antiretroviral-experienced subjects enrolled, 32 were PI-naive and NRTI-experienced while 24 were PI- and NRTI-experienced.
- 88% (21) of the PI-experienced subjects had received ritonavir (standard dose) and 29% (7) had received multiple PIs.
- No age effect was observed in the pharmacokinetic analysis of AUC (p=0.2) or C trough (p=0.7).
Subject Disposition
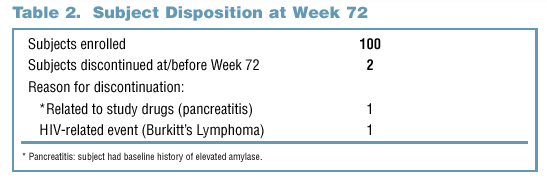
- Of the 100 subjects enrolled in the study, two subjects had discontinued as of Week 72 (Table 2).
Safety
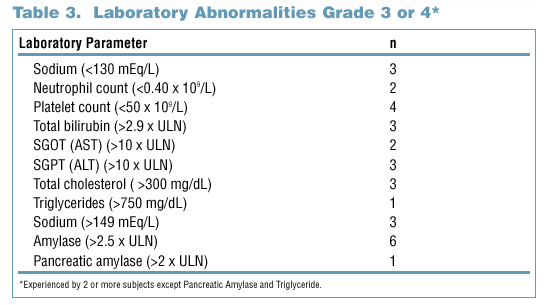
- There were few grade 3/4 laboratory abnormalities, or adverse events of at least moderate severity and of probable or possible relationship to study
drug, at 72 weeks (Tables 3 and 4).
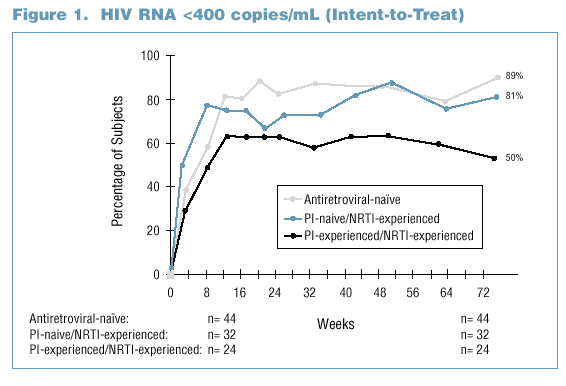
Viral Load Suppression to <400 copies/mL at 72 Weeks
- The proportion of subjects with viral load less than 400 (less than 50) copies/mL at Week 72 was 89% (71%) for antiretroviral-naïve subjects and
68% (55%) for antiretroviral-experienced subjects by an Intent-to-Treat analysis.
- In general, the proportion of subjects below 400 copies/mL was higher in the antiretroviral-naïve group than in the PI- and NRTI- experienced group
beginning at Week 12 and continuing through Week 72 (Figure 1).
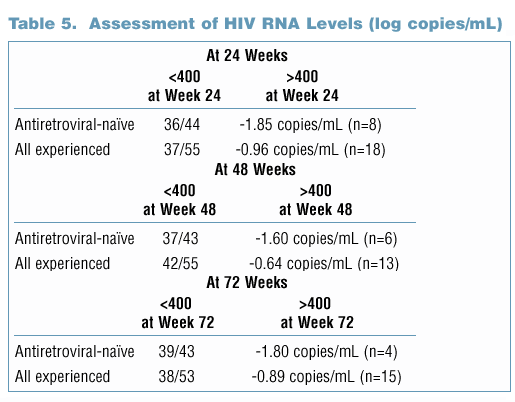
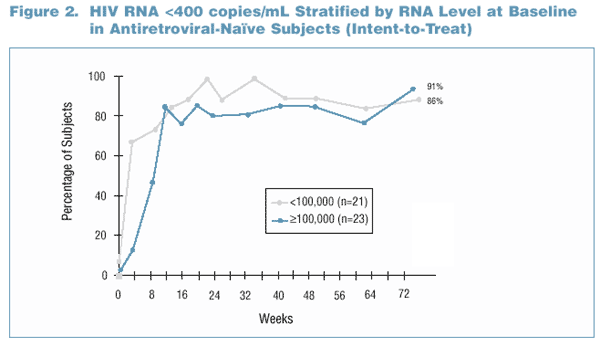
Incidence of Resistance in Antiretroviral-Experienced Subjects
- Baseline resistance data were available for 20 of 24 PI-experienced subjects. Of these, 1 of 3 subjects with >10-fold and 10 of 17 subjects with
<10-fold reduced baseline susceptibility (by phenotypic assay) to lopinavir had HIV RNA <400 copies/mL at Week 72.
Incidence of Resistance in PI-Naïve Subjects
- Genotype was available from 13 of 31 PI-naïve subjects with HIV RNA >400 at Weeks 24, 48, or 60.
- Resistance to Kaletra was defined as any primary or active site mutation in protease (amino acids 8, 30, 32, 46, 47, 48, 50, 82, 84 and 90).2
- Development of protease inhibitor resistance was not observed in PI-naïve subjects.

CD4 Count Response
- Mean CD4 count increase from baseline was 387 cells/mm3
for antiretroviral-naive subjects and 435
cells/mm3
for antiretroviral-experienced subjects
by Week 72.
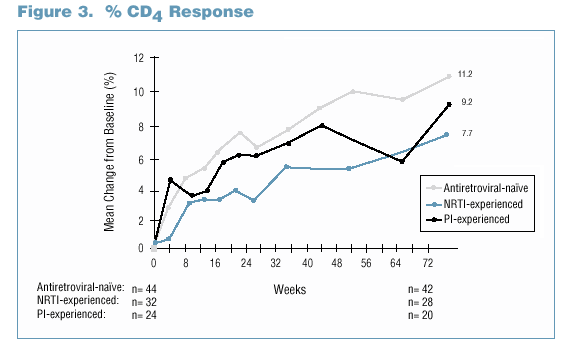
- 39 of 43 subjects (91%) with baseline CD4 count =24% experienced an improvement in immunologic function of at least one category, while 1 of 57 subjects (2%) with
baseline CD4 count >24% experienced a decline in immunologic function.
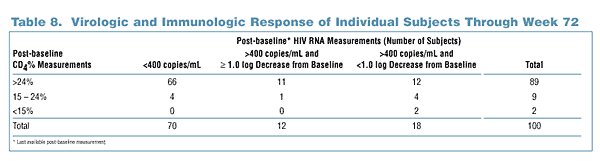
- 23 of 30 subjects (77%) with viral load >400 copies/mL at last available measurement had corresponding CD4 count > 24%.
CONCLUSIONS
- The liquid formulation of Kaletra was well tolerated by HIV-infected children with only one discontinuation related to study drug through 72 weeks.
- There were few adverse events of at least moderate severity and of probable or possible relationship to study drug or grade 3/4 laboratory abnormalities through 72 weeks.
- Kaletra demonstrated excellent antiviral activity with 89% of antiretroviral-naïve subjects and 68% of antiretroviral-treatment-experienced subjects having viral load
<400 copies/mL HIV RNA at Week 72 using an Intent-to-Treat analysis.
- Kaletra demonstrated excellent immunologic activity with 91% of subjects with baseline CD4 count <24% having improved immunologic function by at least one category.
ACKNOWLEDGEMENTS
Fundacion Huesped, Buenos Aires, Argentina Patricia Coll, Carlos Zala
The Hospital for Sick Children, Toronto, Canada Upton Allen
Univ. of Texas Southwestern Medical Center,
Dallas, U.S.A. Octavio Ramilo
St. Luke’s Roosevelt Hospital Center,
New York City, U.S.A. Stephen Arpadi
Children’s Memorial Hospital, Chicago, U.S.A. Ellen Chadwick
University Hospital of Brooklyn, New York City, U.S.A. Edward Handelsman
Max Finland Laboratory of Infectious Disease,
Boston, U.S.A. Stephen Pelton
Abbott Laboratories Jaime Baldner, Richard Bertz,
Carl Deetz, Ann Hsu, Ping Jiang,
Dale Kempf, Renee Reitmeyer, Richard Rode
Pharmaceutical Research Associates, Inc. John DiFulvio, Jeff Knight,
Andres Lategan, Nancy Rivera, Kathleen Walker,
Terry Wheeler
Genotype and Phenotype testing was performed by ViroLogic, Inc.
REFERENCES
1. Bertz R, Lam W, Brun S, et al. Multiple-dose pharmacokinetics
(PK) of ABT-378/ritonavir (Kaletra) in HIV+ subjects. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy,
San Francisco, USA, 1999, (abstract 0327).
2. Hirsch MS, et al. Antiretroviral drug resistance testing in adults with HIV infection: Implications for Clinical Management. International AIDS Society—USA Panel. JAMA 1998; 279: 1984-91.
TOP

Poster
Kaletra (ABT-378/ritonavir) in HIV-Infected Children at 72 Weeks
© 2001
Medical Advocates for Social Justice
Email: info@medadvocates.org










