
 |
Share this Poster with a Colleague
BACKGROUND
Study M98-863 is a large, blinded, randomized, prospective study comparing the activity and safety of lopinavir/ritonavir (LPV/r) plus d4T and 3TC to that of
nelfinavir (NFV) plus d4T and 3TC in antiretroviral (ARV)-naïve subjects. A total of 653 subjects enrolled in the study and were assigned to receive d4T + 3TC and
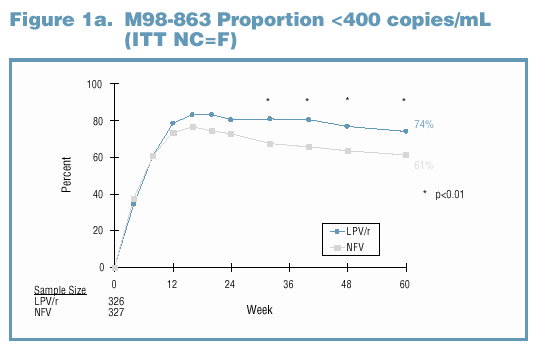
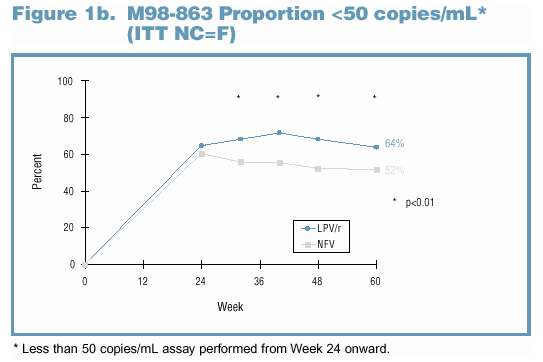
either NFV or LPV/r. Through Week 60, significantly more LPV/r-treated subjects experienced viral suppression than NFV-treated subjects (Figures 1a and 1b).
The differences in efficacy observed in this study may reflect the substantially different inhibitory quotients (IQ, Ctrough/IC50)1
achieved with LPV/r versus NFV.2
A high IQ may also provide a pharmacologic barrier to the development of drug-resistance. The objective of this analysis was to evaluate
differences in the incidence of protease inhibitor (PI) and reverse transcriptase inhibitor (RTI) resistance between the two arms of this controlled
comparative study.
METHODS
Genotypic/Phenotypic Resistance
Samples from all subjects with VL >400 copies/mL at least once at Week 24, 32, 40, 48 or 60 while on the assigned treatment regimen were submitted
for analysis. Genotype (GeneSeq) and phenotype (PhenoSense) were performed by ViroLogic, Inc.
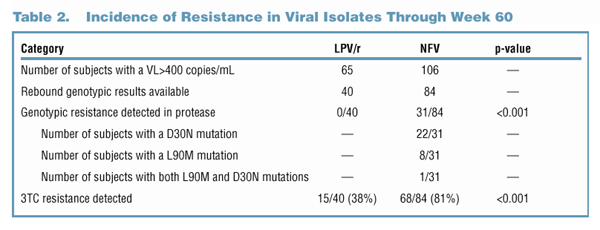
Genotypic resistance to NFV was defined as the development of a D30N and/or an L90M mutation in protease. Genotypic resistance to LPV was defined
as the development of any primary or active site mutation in protease (amino acids 8, 30, 32, 46, 47, 48, 50, 82, 84 and 90). Phenotypic analyses were
performed on all samples obtained from LPV/r-treated subjects to confirm the lack of resistance to LPV. Resistance to 3TC was defined as the presence
of an M184V and/or M184I mutation in reverse transcriptase.
Adherence
Overall adherence was measured by pill counts of protease inhibitor (non-placebo), and was computed as the percentage of pills consumed relative to
the expected number consumed.
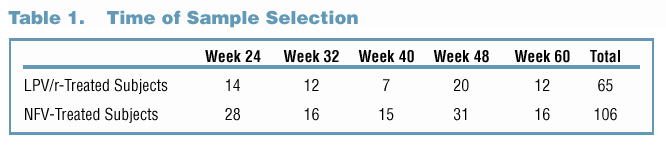
Sample Selection for Analysis
A total of 65 LPV-treated and 106 NFV-treated subjects had at least one VL >400 copies/mL at Week 24, 32, 40, 48 or 60 while on treatment. For
subjects with multiple VLs >400 copies/mL during Weeks 24 through 60, the latest sample for which the genotypic sequence was available was used for
this analysis, unless PI resistance had been identified previously. A summary of sample selection is provided in Table 1.
RESULTS
Lower Incidence of Resistance in LPV/r-Treated Subjects
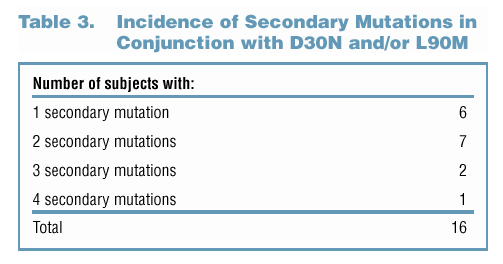
Appearance of Other Mutations in NFV-Treated Subjects Who Developed D30N and/or L90M Mutation
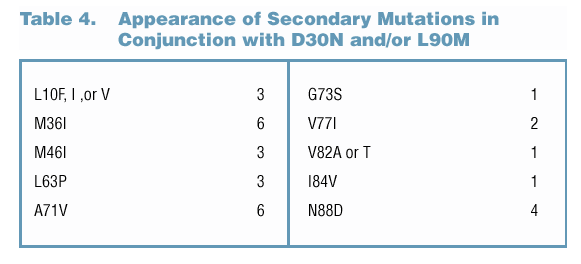
Appearance of Polymorphisms/Secondary Mutations in LPV/r-Treated Subjects
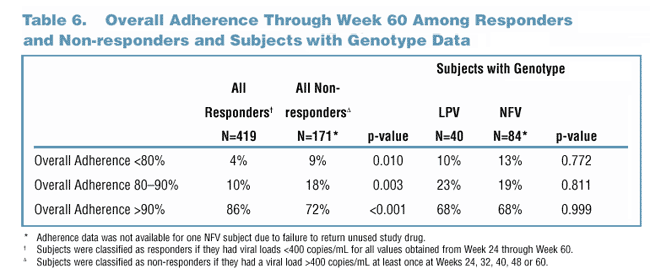
Responders Had Better Adherence Than Non-responders
DISCUSSION
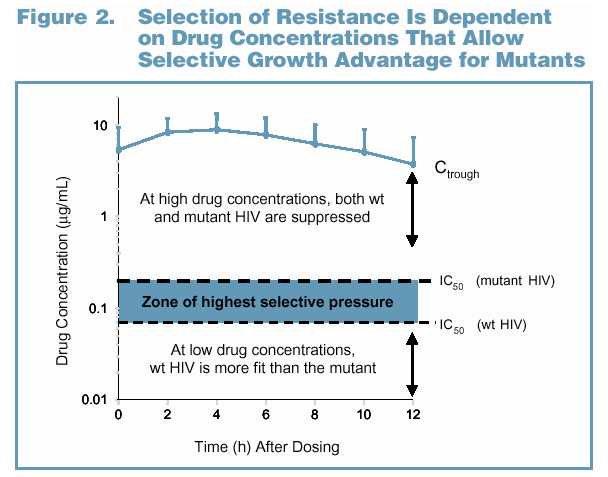
A semi-quantitative pharmacological model may account for the lack
of resistance to lopinavir observed in this study (Figure 2). During
periods of adherence, plasma levels of LPV remain well in excess of
the serum-adjusted IC50 against wild-type HIV (high inhibitory
quotient), and viral replication of both the wild-type virus and any
pre-existing viral mutants are likely to be suppressed. Drug
concentrations that lie between the IC50 values for the wild-type and
mutant viruses are expected to provide the greatest selective
replication advantage for the mutant (zone of highest selective
pressure). During periods of non-adherence, plasma drug levels
decline through the zone of highest selective pressure. As drug
levels continue to decline below this concentration zone, overall
replication increases, and, in the absence of drug, the wild-type virus
has a fitness advantage over any mutants. Understanding the
selection of resistance in vivo requires an estimation of the time
during which significant selective pressure exists (i.e., how frequently
and how rapidly plasma drug concentrations decline through the
zone of highest selective pressure).
Single mutants display <3-fold reduced susceptibility to lopinavir.
4,5
Based on steady-state pharmacokinetic determinations, the median
estimated time for plasma levels of LPV to decay to the upper
boundary of zone of highest selective pressure is 20.5 hours. This time would approximate missing a scheduled LPV/r BID dose by 8 hours. By this time,
plasma concentrations of ritonavir have declined to levels that no longer adequately inhibit the metabolism of lopinavir. Thus, as concentrations of lopinavir
enter the zone of highest selective pressure, the clearance of lopinavir has increased substantially (short half-life). Thus, the estimated median time for LPV
concentrations to decay through the zone of highest selective pressure is approximately 3.5 hours (range 2.5-5 hours). Since viral maturation through
proteolytic processing by active (uninhibited) wild-type HIV protease is not instantaneous (estimated half-life up to 1.5 hours for production of mature, infectious
particles)6
and the kinetics of HIV protease containing primary mutations can be substantially impaired compared to wild-type protease,7
the selective
production of infectious virus containing primary resistance mutations may be minimal during this period.
CONCLUSIONS
REFERENCES
1. Neu, H. The inhibitory quotient. A method for interpreting minimum inhibitory concentration data. JAMA, 1981; Oct. 2, 246 (14): 1575-8.
2. Bertz, R et al. Multiple Dose Pharmacokinetics (PK) of ABT-378/ritonavir (ABT-378/r) in HIV+ Subjects. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy. San Francisco, USA, 1999 (Abstract 0327).
3. Hirsch, M.S. et al. Antiretroviral drug resistance testing in adults with HIV infection: Implications for clinical management. International AIDS Society-USA Panel. JAMA 1998;279: 1984-91.
4. Molla A, Vasavanonda S, Kumar G, et al. Human serum attenuates the activity of protease inhibitors toward wild-type and mutant human immunodeficiency virus. Virology 1998; 250: 255-62.
5. Carrillo A, Stewart K, Sham HL, et al. In vitro selection and characterization of human immunodeficiency virus type 1 variants with increased resistance to ABT-378, a novel protease inhibitor.
J. Virology 1998; 72:7532-7541.
6. Kaplan, A, Manchester M, and Swanstrom R. The activity of the protease of human immunodeficiency virus type 1 is initiated at the membrane of infected cells before the release of viral proteins and is required for release to occur with
maximum efficiency. J. Virology 1994; 68:6782-6786.
7. Schock HB, Garsky VM, and Kuo LC. Mutational anatomy of an HIV-1 protease variant conferring cross-resistance to protease inhibitors in clinical trials-compensatory modulations of binding and activity. J. Biol. Chem 1996; 271:31957-31963.
Adherence and Viral Exposure Were Similar Between Treatment Groups Among Subjects with Genotype Data
© 2001
Medical Advocates for Social Justice
Email: info@medadvocates.org