|
Share this Poster with a Colleague Kaletra Library Service Home |
BACKGROUND
Kaletra (formerly known as ABT-378/r, lopinavir/ritonavir) is a protease inhibitor (PI) that has shown significant antiviral activity and tolerability in clinical trials to date.
Lopinavir is exquisitely sensitive to pharmacokinetic enhancement by ritonavir, resulting in substantially increased LPV drug exposure, even at low ritonavir doses. At the dosage selected for phase III clinical trials, 400 mg LPV/100 mg ritonavir BID (as 3 co-formulated capsules BID). The mean LPV Ctrough/EC50 ratio (Inhibitory Quotient or IQ) for wild-type HIV is >75 when dosed at 400/100 mg BID, potentially providing superior antiviral activity and a pharmacologic barrier to the emergence of viral resistance.1
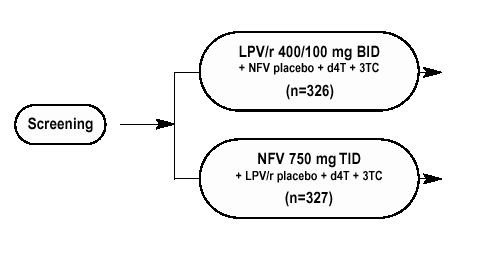
M98-863 is a double-blind, randomized trial comparing the safety and antiviral activity of Kaletra (LPV/r)+d4T+3TC vs. that of nelfinavir (NFV)+d4T+3TC in antiretroviral naive subjects. It is being conducted at 93 centers in 13 countries, covering 5 continents and it is one of the largest prospective, randomized clinical trials of protease inhibitor therapy.
The primary efficacy analyses included:
This update is a summary of results through Week 60.
METHODS
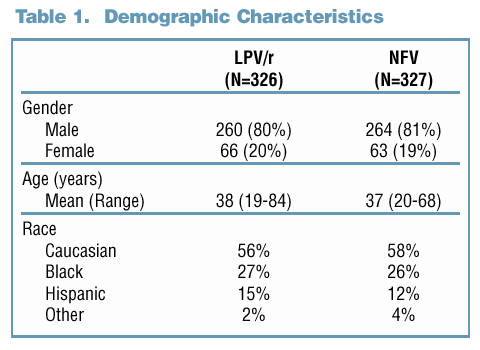
Entry Criteria
Study Design and Analysis
653 antiretroviral-naďve subjects were randomized to receive either:
RESULTS
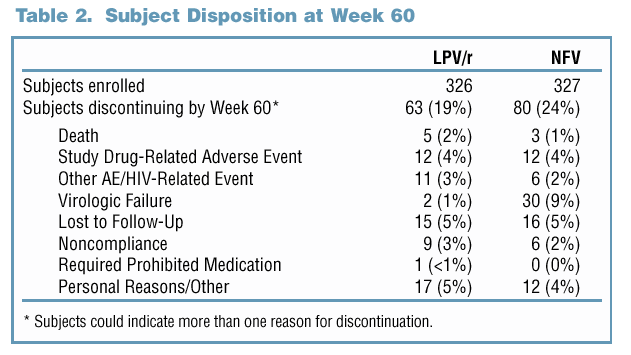
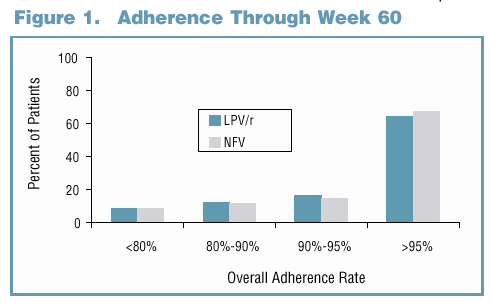
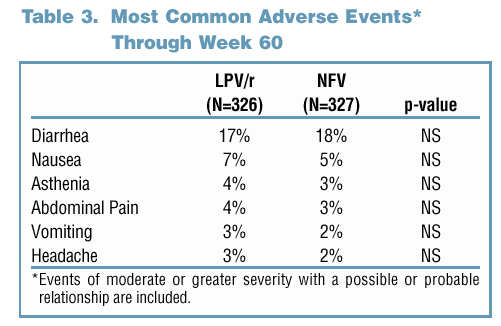
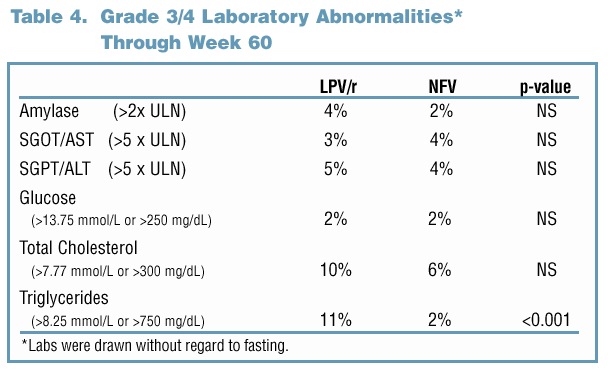
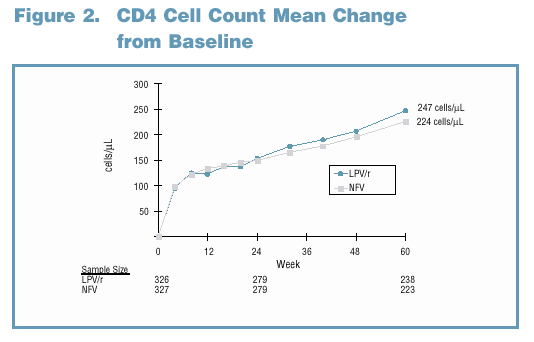
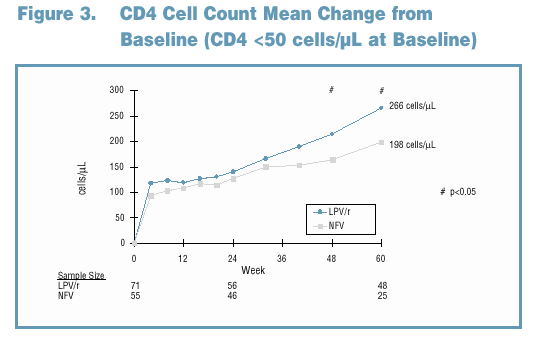
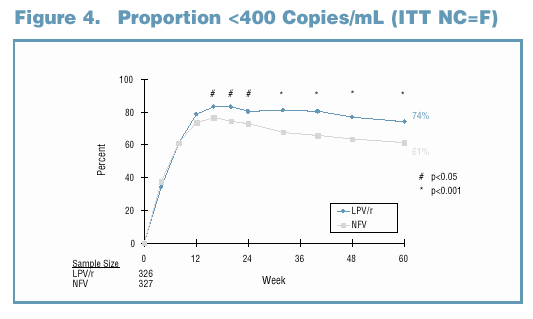
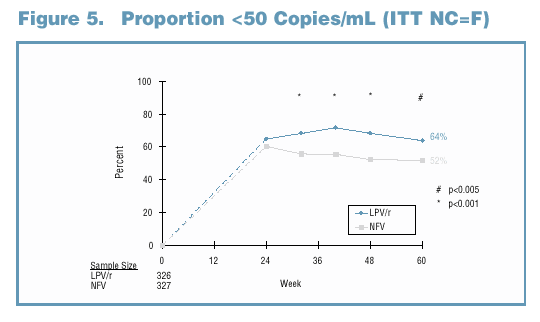
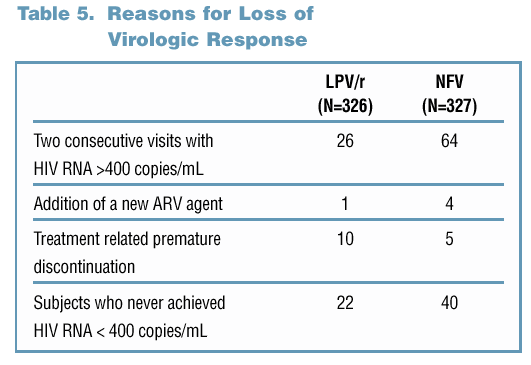
81% and 65% of LPV/r and NFV-treated subjects maintained a viral response through Week 60
INCIDENCE OF PI RESISTANCE
Of subjects with a viral load >400 copies/mL through Week 60, 38% of the LPV/r-treated subjects vs. 81% of the NFV-treated subjects had developed resistance to 3TC (p<0.001).
CONCLUSIONS
REFERENCES
1. Bertz R, Lam W, Brun S, et al. Multiple-dose pharmacokinetics (PK) of LPV/ritonavir (LPV/r) in HIV+ subjects. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, USA, 1999 (abstract 0327).
ACKNOWLEDGEMENTS
|
Anthony M. Allworth, M.D. Frederick Altice, M.D. Keikawus Arasteh, M.D. Jose Arribas, M.D. Carlos Barros, M.D. Jeffrey Beal, M.D. Gildon Beall, M.D. John David Brand, M.D. Andrew Badley, M.D. Paul Cimoch, M.D. Bonaventura Clotet Sala, M.D. Calvin Cohen, M.D. Timothy Cooley, M.D. Prof. Jean-Francois Delfraissey Charles Farthing, M.D. Gerd Faetkenheuer, M.D. Judith Feinberg, M.D. Margaret Fischl, M.D. Martin Fisher, M.D. Markus Flepp, M.D. Joel Gallant, M.D., M.P.H. Joseph Gathe, M.D. Jan Gerstoft, M.D. |
Mitchell Goldman, M.D. Juan Gonzalez-Lahoz, M.D. Frank M. Graziano, M.D. Stephan Green, M.D. Howard A. Grossman, M.D. David Haas, M.D. Stephen Hauptman, D.O. Charles Hicks, M.D. Andrzej Horban, M.D. James Horton, M.D. Newton Hyslop, M.D. Dave Johnson, M.D. Margaret Johnson, M.D. Robert Kalayjian, M.D. Powel Kazanjian, M.D. Jay Kostman, M.D. Richard Lalonde, M.D. Harry Lampiris, M.D. Francois LaPlante, M.D. Jeffrey Lennox, M.D. Roberta Luskin-Hawk, M.D. Simon Mallal, M.D. Lars Mathiesen, M.D. |
Joao Mendonca, M.D. Robert Myers, M.D. Peter Miao, M.D. Donna Mildvan, M.D. Steve Miller, M.D. Julio Montaner, M.D. Prof. Yves Mouton Andy Pavia, M.D. Court Pedersen, M.D. Gerald Pierone, M.D. Richard Pollard, M.D. Anton Pozniak, M.D. Anita Rachlis, M.D. Frank Rhame, M.D. Peter Ruane, M.D. Rafael Rubio, M.D. Prof. Adrien G. Saimot James H. Sampson, M.D. Ian Sanne, M.D. Jorge Santana Bagur, M.D. Daniel Seekins, M.D. Prof. Daniel Sereni Gladys Sepulveda, M.D. |
Sheetal Sharma, M.D. Renslow Sherer, M.D. Leon Smith, M.D. Kathleen Squires, M.D. Schlomo Staszewski, M.D. Roy T. Steigbigel, M.D. Corklin R. Steinhart, M.D. Albrecht Stoehr, M.D. Donna E. Sweet, M.D. Karen Toshima, M.D. Albert Theisen, M.D. Rejean Thomas, M.D. James A. Thommes, M.D. Melanie A. Thompson, M.D. Artur Timermann, M.D. Pompeyo Viciana, M.D. Daniel Vittecoq, M.D. Sharon Walmsley, M.D. Prof. Jonathan Weber Lutwin Weitner, M.D. I. P. van der Westhuizen, M.D. David Wheeler, M.D. David P. Wright, M.D. Bienvenido G. Yangco, M.D. |
© 2001
Medical Advocates for Social Justice
Email: info@medadvocates.org