|
Share this Poster with a Colleague Kaletra Library Service Home |
BACKGROUND
Lopinavir (LPV) is an HIV protease inhibitor (PI) that is co-formulated with ritonavir, which functions as an inhibitor of cytochrome P450 3A. Even at low ritonavir doses, there is a substantial increase in LPV exposure. At a dosage of 400 mg of LPV/100 mg ritonavir twice daily (3 co-formulated tablets BID), ritonavir concentrations are below those required for antiviral activity.1 By contrast, the mean LPV Ctrough/EC50 ratio (Inhibitory Quotient or IQ) for wild-type HIV is >75 when dosed at 400/100 mg twice a day, potentially providing a barrier to emergence of viral resistance and activity against resistant virus. Lopinavir/ritonavir (LPV/r, marketed as Kaletra) has been studied in both anti-retroviral naive and experienced HIV-infected patients. However, few long-term data are available on continued safety and efficacy. The M97-720 study is an ongoing phase II double-blind trial of LPV/r in combination with d4T and 3TC in antiretroviral-naive patients. This was the first trial of LPV/r in HIV-infected patients and hence provides the longest duration of follow-up for patients treated with LPV/r. This poster presents data on safety and efficacy through 144 weeks.
METHODS
Entry Criteria
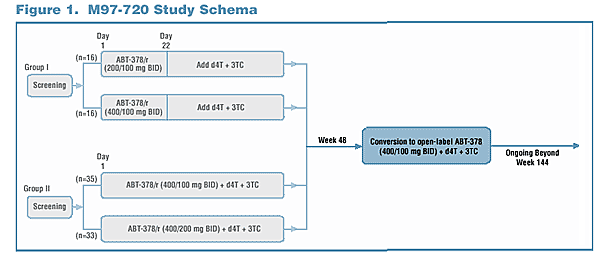
Study Design and Analysis
Antiviral Activity
RESULTS
Baseline Characteristics
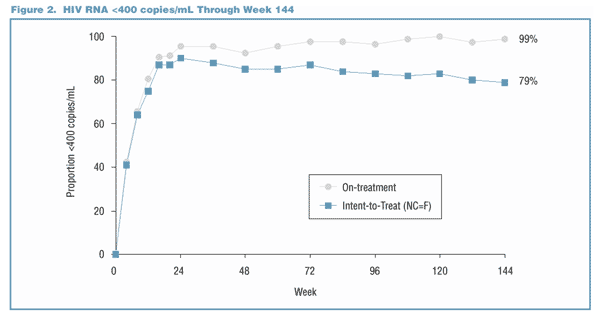
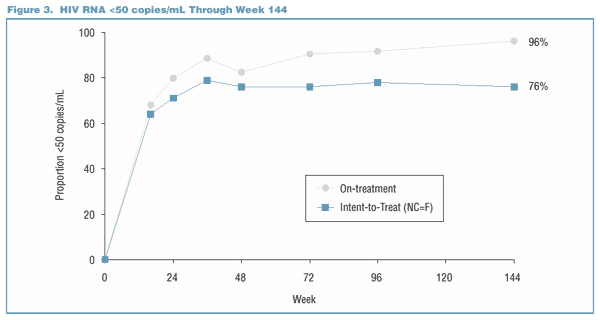
Viral Load Suppression Below the LLQ Through Week 144
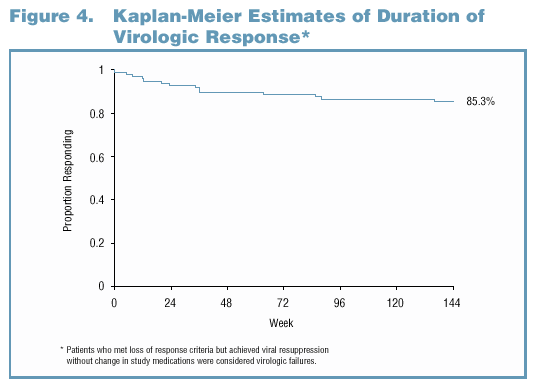
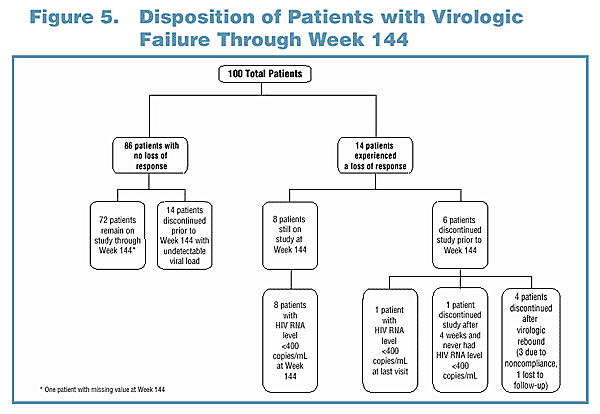
Duration of Virologic Response Analysis
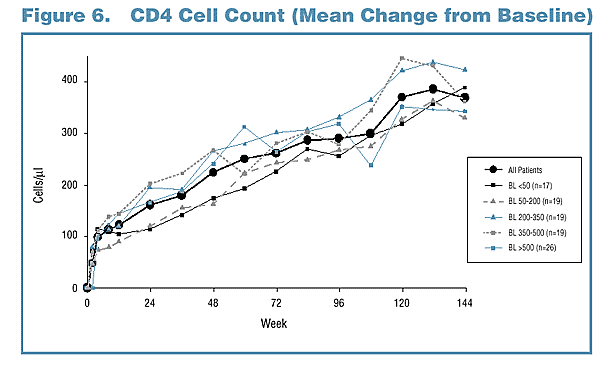
CD4 Cell Count Response
U.S. Department of Health and Human Services (DHHS) Guidelines
CONCLUSIONS
ACKNOWLEDGEMENTS
M97-720 Study Subjects
Covance Central Laboratory Services
AIDS Research Consortium of Atlanta Sullivan M
Beth Israel Deaconess Medical Center Fitch H
Cornell Clinical Trials Unit Stroberg T
Duke University Medical Center Giner J, Harmon L
Northwestern University Bruce J
Pacific Oaks Research Perry B, Walker S
Rush Presbyterian St. Luke's Medical Center Narkiewicz E
Thomas Street Clinic Sepcie B
University of Colorado Canmann S, Putnam B, Ray MG
University of North Carolina at Chapel Hill Ngo L
PPD Development McCarley S, Nicks B, Wheat R
Abbott Laboratories Kempf D, Potthoff A, Rode R, Sheehan K, Yang G
REFERENCES
1. Bertz R, Lam W, Brun S, et al. Multiple-dose pharmacokinetics (PK) of LPV/ritonavir (LPV/r) in HIV+ subjects. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, USA, 1999 (abstract 0327).
2. Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. Updated 23 April 2001.*Amplicor HIV-1 Monitor is a trademark of Roche Molecular Diagnostics
© 2001
Medical Advocates for Social Justice
Email: info@medadvocates.org