
 |
Share this Poster with a Colleague
[Introduction] [M97-720 Study Design] [M97-720 Results] [M97-765 Design]
[M97-765 Results] [Conclusion at 36 Weeks] [Acknowledgements]
Introduction: ABT-378/r |
|
|
|
Study Design |
|
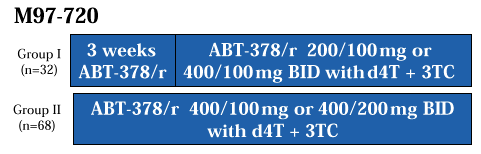
|
|
Results |
|
|
Reasons for Discontinuation:
|
|
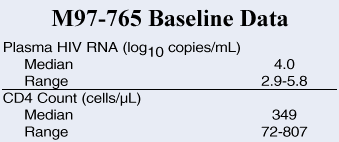
Study Design |
|

|
|
Results |
|
Reasons for discontinuation:
|
|
Conclusions at 36 Weeks |
|
|
|
Acknowledgements |
|
| Beth Israel Deaconess Medical
Center -
Helen Fitch, RN, Carolyn Koziol, RN
University of Colorado Health Sciences Center - Sally Canmann, RN, Bev Putnam, RN, ANP, M. Graham Ray, MSN, ANP San Francisco General Hospital - Dorie Heeren University of North Carolina-Chapel Hill - Barbara Longmire, RN, Cheryl Marcus, RN, Linh Ngo, FNP University of Cincinnati - Jarlath Black, RN, Pam Daniel, RN, Jill Leonard, RN Cornell Clinical Trials Unit - Todd Stroberg, RN,Tricia Sarraco, RN Duke University Medical Center - Julieta Giner, RN,ACRN, Les Harmon, RN, MSN,ANP Rush Presbyterian St.Luke's Medical Center - Elke Narkiewicz, RN, BSN Northwestern University - Jim Bruce, RN,ACRN, Pam Donath, RN, Siddharth Padia University of Pittsburgh - Rosella Rosener, RN Brigham and Women's Hospital - Martha VanDerVleit Pacific Oaks Research - Kai Gu, RN, Donna Morales, RN AIDS Research Consortium of Atlanta - Tim Enstrom, PA-C Infectious Disease Physicians - Stephen Poretz, Ja'Ree Thompson, CRC Thomas Street Clinic - Barbara Sepcie, RN PPD Development, Inc. - Sandy McCarley, RN, Shannon Jackson, RN, Alice Donnelly |
Abstract prepared by Abbott Laboratories
From the 39th ICAAC
![]()
|
|
|
|
|
ABT-378/r: 36 Week
Phase II Update |