|
Share this Poster with a Colleague Kaletra Library Service Home |
BACKGROUND
The long-term efficacy of antiretroviral regimens can be compromised by the emergence of resistant virus. As a result, optimal sequencing of antiretroviral regimens as part of an overall treatment strategy is critical to maximize the duration of effective antiretroviral therapy. Kaletra is a coformulation of lopinavir (LPV) and ritonavir (r), which inhibits LPV metabolism by CYP3A, providing increased plasma levels of LPV. Kaletra differs from other approved protease inhibitors in that its plasma drug levels far exceed concentrations estimated to inhibit wild-type HIV replication in vivo, and to a lesser and variable extent, those concentrations estimated to inhibit HIV in patients who have failed prior PI therapy. Consistent with these pharmacodynamics, the proportion of ARV-naive patients receiving Kaletra with d4T and 3TC with HIV RNA <400 copies/mL (99% on treatment, 79% ITT through 144 weeks, White 2001) was higher than that of PI-experienced patients receiving Kaletra with nevirapine and 2 NRTIs (81% on treatment, 63% ITT through 96 weeks, Feinberg 2000).
OBJECTIVES
The objective of this analysis was to determine the sequence of LPV/r use resulting in maximal duration of viral suppression. Since no prospective studies evaluating sequencing strategies that include LPV/r have been reported, data from two phase II studies of LPV/r were analyzed to assess the effect of prior PI therapy on overall duration of viral suppression on a LPV/r-based regimen. The results of this retrospective analysis may have implications for sequencing strategies involving LPV/r.
METHODS
Analysis A
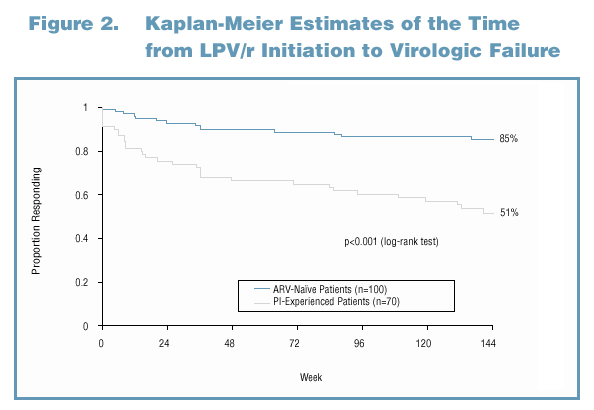
PI-experienced patients were compared to ARV-naive patients with respect to the time from LPV/r initiation to virologic failure. For patients who achieved viral load (VL) <400 copies/mL while on LPV/r, virologic failure was defined as the first of two consecutive VLs to rebound above 400 copies/mL. For patients who never achieved VL <400 copies/mL, virologic failure was defined as the first day of LPV/r dosing. Patients who discontinued without experiencing virologic failure were censored at the time of discontinuation.
Analysis B
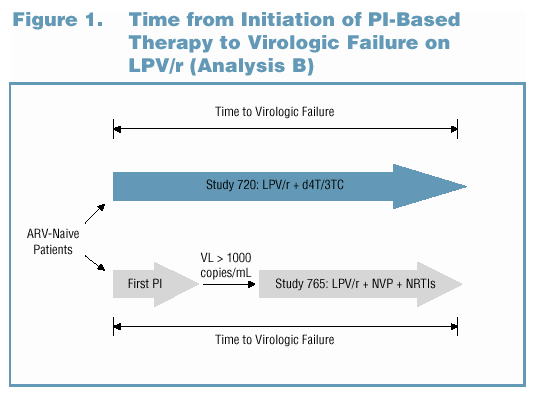
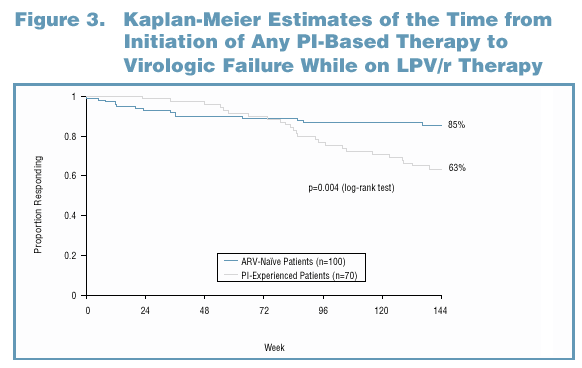
The preceding analysis does not account for the duration of first-line therapy with a PI other than LPV/r. Therefore, to examine whether using another PI prior to LPV/r provides incremental benefit (with respect to overall duration of virologic suppression), the time from initiation of any PI-based therapy to virologic failure while on LPV/r was analyzed. This definition of the time to virologic failure is illustrated in Figure 1. As in an analysis by Levy et al. (Levy 2001), the median time to loss of virologic response was estimated by extrapolating from the failure rate during the 3rd year of treatment (Weeks 96-144).
Analysis C
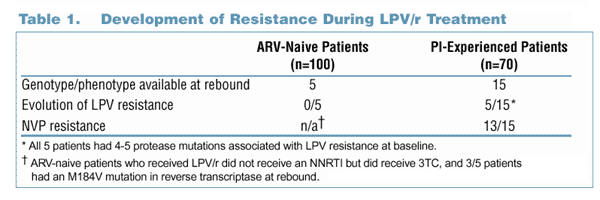
To explore the impact of these sequencing strategies on the development of resistant virus, genotypic and phenotypic data were analyzed. Genotypic (population sequencing) and Phenotypic (Antivirogram or PhenoSense method) analyses were performed at baseline for all subjects in Study 765 and at virologic rebound while on LPV/r-based therapy for subjects in both studies. Evolution of LPV resistance was defined as the appearance of any new PI resistance-associated mutation during virologic rebound.
Analysis D
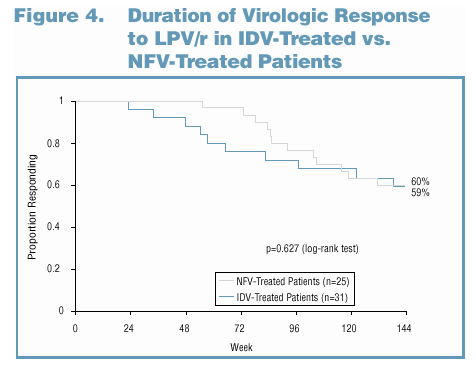
Indinavir and nelfinavir were the most frequently used PIs among patients entering Study 765. To assess the effect of indinavir (IDV) or nelfinavir (NFV) use on subsequent response to a LPV/r-based regimen as second-line therapy, the time to loss of virologic response on LPV/r-based therapy was compared between the groups of IDV-treated patients (n=31) and NFV-treated patients (n=25) in Study 765. For all analyses of the time to loss of virologic response, Kaplan-Meier estimates were computed, and comparisons between groups were made using the log-rank test. Adherence was measured by pill count of returned capsules of LPV/r. Overall adherence was measured as the percentage of capsules taken relative to the number expected to be taken. Differences in adherence rates were analyzed using a one-way analysis of variance.
RESULTS
Analysis A: ARV-Naive Patients Achieved a More Durable Response on LPV/r-Based Therapy than Single PI-Experienced Patients
Analysis B: Using Another PI as First-Line Therapy Instead of LPV/r Did Not Provide Long-Term Advantage in the Duration of Response
Analysis C: Viral Resistance After Virologic Rebound on LPV/r
Analysis D: Patients on Failing NFV or IDV Regimens
Had Comparable Responses to
LPV/r-Based Therapy
DISCUSSION
Patients enrolling in Study 765 had a mean duration of first-line therapy of 18 months, with a range of 6 months to 4 years. Reports on the durability of first-line PI-based therapy indicate that most patients discontinued their first-line therapy or were not maintaining virologic suppression 12 to 15 months after therapy initiation (Ledergerber 1999, Lucas 1999, Butcher 2000, Levy 2001), suggesting that the duration of first-line therapy for subjects enrolling in Study 765 is comparable to that typically seen in ARV-naive patients initiating PI-based regimens.
In this analysis, ARV-naive patients had a significantly longer duration of response than single PI-experienced patients, whether the duration of first-line therapy was accounted for in the analysis (Figure 3) or not (Figure 2).
This analysis has important limitations. Foremost, it is a retrospective analysis of two clinical trials. The definitive method of investigating the question of whether there is a benefit to reserving LPV/r for use after the failure of a first-line PI-based regimen (instead of using LPV/r as first-line therapy) would be to conduct a prospective clinical trial in which ARV-naive patients were randomized either to receive first-line therapy with LPV/r or to receive second-line therapy with LPV/r after failure of a first-line PI-based regimen. Another limitation is that the definition of virologic failure (consecutive viral loads demonstrating rebound above 400 copies/mL) may not be clinically relevant for patients with subsequent viral resuppression without a change in regimen.
Sensitivity analyses can be used to explore these limitations.
Sensitivity Analysis 1
Rationale: In a prospective trial with ARV-naive patients randomized either to receive LPV/r as first-line therapy or to receive another PI as first-line therapy with LPV/r as second-line therapy, it is likely that some patients who were randomized to receive a PI other than LPV/r would maintain virologic response for the entire 3-year period covered by the analyses described here. (That is, the enrollment criteria for Study 765 may "select" for patients who have adherence difficulties or who are otherwise less likely to respond well to ARV therapy.) In such a situation, the Kaplan-Meier estimates for PI-experienced patients would be higher than those shown in Figure 3.
Details: Determine how many additional such patients would have been necessary so that the Kaplan-Meier estimates no longer favor patients who used LPV/r as first-line therapy.
Results: If 101 additional such patients (who maintained response on their first-line PI for at least 144 weeks) were included, the Kaplan-Meier estimates at Week 144 for patients who used a PI other than LPV/r would match those of ARV-naive patients shown in Figure 3 (85%).
Discussion: An additional 101 patients maintaining response on their first-line PI for 144 weeks corresponds to the assumption that over 60% of patients initiating first-line PI-based therapy can be expected to maintain virologic suppression on their first-line regimen for over 3 years. This assumption is unlikely-several studies suggest that after only 2 years of therapy, no more than 40% of ARV-naive patients who initiated PI-based therapy with drugs such as IDV and NFV were still on their first-line PI-based regimen with suppressed viral loads (Ledergerber 1999, Butcher 2000, Levy 2001). Therefore, it appears that the difference shown in Figure 3 is not completely explained by the possibility that the enrollment criteria for Study 765 may have selected for patients less likely to experience virologic success than the general patient population.
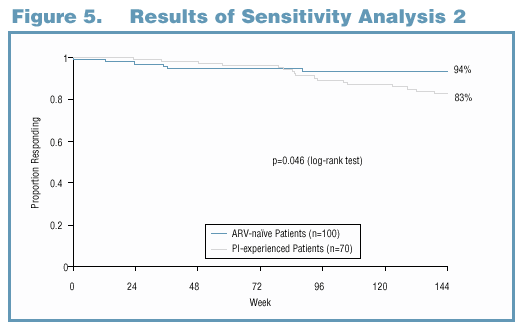
Sensitivity Analysis 2
Rationale: For some patients, the definition of virologic failure may not be clinically relevant.
Details: Patients who met the virologic failure criteria but subsequently experienced viral resuppression (without change in regimen) and maintained it through 144 weeks of PI-based therapy were censored (instead of being considered virologic failures) at the time they met virologic failure criteria.
Results: Using the revised definition of virologic failure, patients who used LPV/r as first-line therapy demonstrated significantly longer time to virologic failure compared to patients who initiated with a PI other than LPV/r and used LPV/r as second-line therapy, even after accounting for the duration of the first-line PI therapy (p=0.046, log-rank test, Figure 5).
The results of these sensitivity analyses support the hypothesis that there is no obvious durability benefit to using a PI other than LPV/r as first-line therapy.
CONCLUSIONS
REFERENCES
White AC, et al. Lopinavir/ritonavir (Kaletra) in antiretroviral-naive HIV+ patients: 144-week follow-up. 1st IAS Conference on HIV Pathogenesis and Treatment, Buenos Aires, July 2001.
Feinberg J, et al. Durable suppression of HIV RNA after two years of Kaletra (ABT-378/ritonavir) therapy in single PI-experienced patients. 5th International Congress on Drug Therapy in HIV Infection, Glasgow, October 2000.
Levy R, et al. Low two-year risk of virologic failure with first regimen HAART. 8th CROI, Chicago, February 2001.
Ledergerber B, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Lancet, 1999. 353: 863 868.
Lucas G, et al. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Int Med, 1999. 131(2):81-87. Butcher D, et al. Differences in duration of therapy and viral suppression rates in different therapy sequencing scenarios: PI to NNRTI vs. NNRTI to PI. 40th ICAAC, Toronto, September 2000.
Kemper C, et al. Sequencing of protease inhibitor therapy: insights from an analysis of HIV phenotypic resistance in patients failing protease inhibitors. AIDS 2001, 15:609-615.
© 2001
Medical Advocates for Social Justice
Email: info@medadvocates.org